ARTICLE
Vol. 133 No. 1509 |
Quality assessment of a large primary GP skin cancer service in Auckland, New Zealand
Non-melanoma skin cancers (NMSC) are the most commonly diagnosed malignancies worldwide.
Full article available to subscribers
Non-melanoma skin cancers (NMSC) are the most commonly diagnosed malignancies worldwide. To date, there is very limited data on NMSC incidence (not recorded by the New Zealand Cancer Registry) and treatment costs in New Zealand. Late last century, New Zealand papers from 1982 and 1998 reported an incidence of 231 and 781 per 100,000 people for BCC, and 124 and 377 per 100,000 people for SCC.1,2 In 2018, it is estimated that 229,867 keratinocytic cancers will be diagnosed in New Zealand; with an age standardised rate of 1,385 basal cell carcinomas and 522 squamous cell carcinomas per 100,000 people.3,4 In light of this data, it is clear that NMSC is a significant and increasing burden in New Zealand. Estimates of New Zealand’s overall NMSC incidence range from 1,749 to 1,906.5 per 100,000. In comparison, UK data reports NMSC incidence of 15 to 154 per 100,000. It is clear that New Zealand leads the global skin cancer epidemic, and increased resources and novel ways are required to address this.4–6
In an Australian retrospective audit, the total cost of NMSC treatment was projected to increase by 22% between 2010 and 2015.7 Considering New Zealand’s similarly high NMSC incidence and population characteristics, it is expected that the New Zealand healthcare sector must also increase their spending beyond the NZD$51.4 million spent in 2007/2008 on treating NMSC alone.5 Therefore, it is imperative that the New Zealand health system develops treatment strategies and increase resource provision to deal with the rising numbers of NMSC diagnoses.
Likewise, melanoma is a significant source of morbidity and mortality in New Zealand. This is reflected in the incidence rate of 41.2 per 100,000 per year (Liang et al 2010), compared to the melanoma incidence rates in US, UK, Sweden and Norway ranging from 19.8 to 31.0 per 100,000 per year (Whiteman et al 2016).8,9 This equates to an increased burden on many areas of the health sector with associated increased financial and workforce implications, especially considering the extensive burden of melanoma on New Zealand’s population.
The Waitemata District Health Board (WDHB) Skin Service has implemented an innovative approach to the management of skin cancer by triaging appropriate excisions to specialist-trained general practitioners (GPSI). Overall, GPSIs possess the ability to reduce patient waiting times, workload and financial burden on secondary/tertiary care and assist in earlier detection and treatment of invasive skin lesions.
The aim of this study was to conduct a retrospective audit of the performance of WDHB GPSIs. The primary outcome was to determine their positive margin rate, with secondary outcome measures being infection rates and time to definitive treatment. We then compared these results with international standards from previously published data on general practitioners performing simple surgery.
Method
Sample
A retrospective audit was conducted on all excisions (n=2,705) performed between 1 January 2016 to 31 December 2016 by surgically trained GPs (13 general practitioners, GPSIs) under the WDHB GPSI programme. Electronic patient records were accessed via Clinical Portal to review histology reports, microbiology reports, prescribing information and clinic letters where appropriate and available.
All lesions excised during the one-year period were included in this analysis, while biopsies (incisional, punch) were excluded. If a lesion had a diagnostic excision biopsy first then re-excised at a later date, it was recorded once in our analysis for the definitive procedure (wide local excision). If a lesion was biopsied and not excised for any reason within our analysis period, it was excluded.
Benign and malignant classification
Lesions were classified as malignant or benign based on the histology report. Our criteria for a lesion to be malignant was: 1) all basal cell carcinoma (BCC), 2) invasive squamous cell carcinoma (SCC), 3) melanoma in situ (MIS) or invasive melanoma (MM), 4) scars from a previously excised malignant lesion which met WLE criteria for the index lesion, 5) other clinically malignant lesions, eg, Merkel cell carcinoma (MCC), rare cutaneous malignancies (RCM) etc. A separate category of ‘in situ’ lesions was recorded for SCC in situ (Bowen’s disease). All other lesions were classified as benign (lesions that are indolent, pre-malignant and/or confined to the epidermis, not including MIS) or non-invasive such as: Benign naevi, actinic keratoses, seborrheic keratoses, solar lentigo, keratoacanthoma, haemangioma, dermatofibroma and neurofibroma. A breakdown for this is available in Table 1.
Margins
Non-melanoma skin cancers (n=1,486), not including SCC in situ, were evaluated for completeness of excision in our analysis. Margins were not assessed for invasive melanoma and melanoma in situ as excisions would either be an excisional biopsy, for which a close or positive margin should not be a negative outcome, or a wide local excision, where acceptable margins differ to NMSC. Margins were not assessed for benign lesions.
Margins for NMSCs were assessed according to the following criteria: ‘incomplete excision’ as containing a positive margin along any border of the excision; and ‘complete excision’ containing all margins of healthy tissue around the lesion. ‘Complete excisions’ were further quantified into ‘closely excised lesions’ which contained <1.0mm of="" healthy="" tissue="" on="" either="" deep="" or="" radial="" margin(s),="" and="" ‘definitively="" excised="" lesions’="" which="" contained="">1.0mm of healthy tissue on all margins around the excised lesion. This is the margin criteria reported by McLaughlin et al (2017).10</1.0mm>
Infection rate
Post-operative infections were a secondary outcome measure in this analysis. Patient clinic follow-up notes were not available on the hospital database, so proxy measures were used to estimate post-operative infection rate: 1) if an antibiotic was prescribed between 3–31 days after the lesion was excised, and/or 2) if wound swab showed pathologic bacterial growth with concurrent antibiotics being prescribed; a post-operative infection was diagnosed. The prescribed antibiotics considered consistent with a skin infection were: flucloxacillin, amoxicillin, amoxicillin with potassium clavulanate, ciprofloxacin, co-trimoxazole, cephalexin monohydrate, cefaclor monohydrate and doxycycline. Finally, if a swab grew no bacteria but the practitioner prescribed antibiotics, this was not considered an infection as we believe that a significant postoperative wound infection would have a positive wound swab result.
Time to treat
Time to treat was defined as the number of days from when the lesion was registered with the WDHB Skin Cancer Service to the date of surgery.
Results
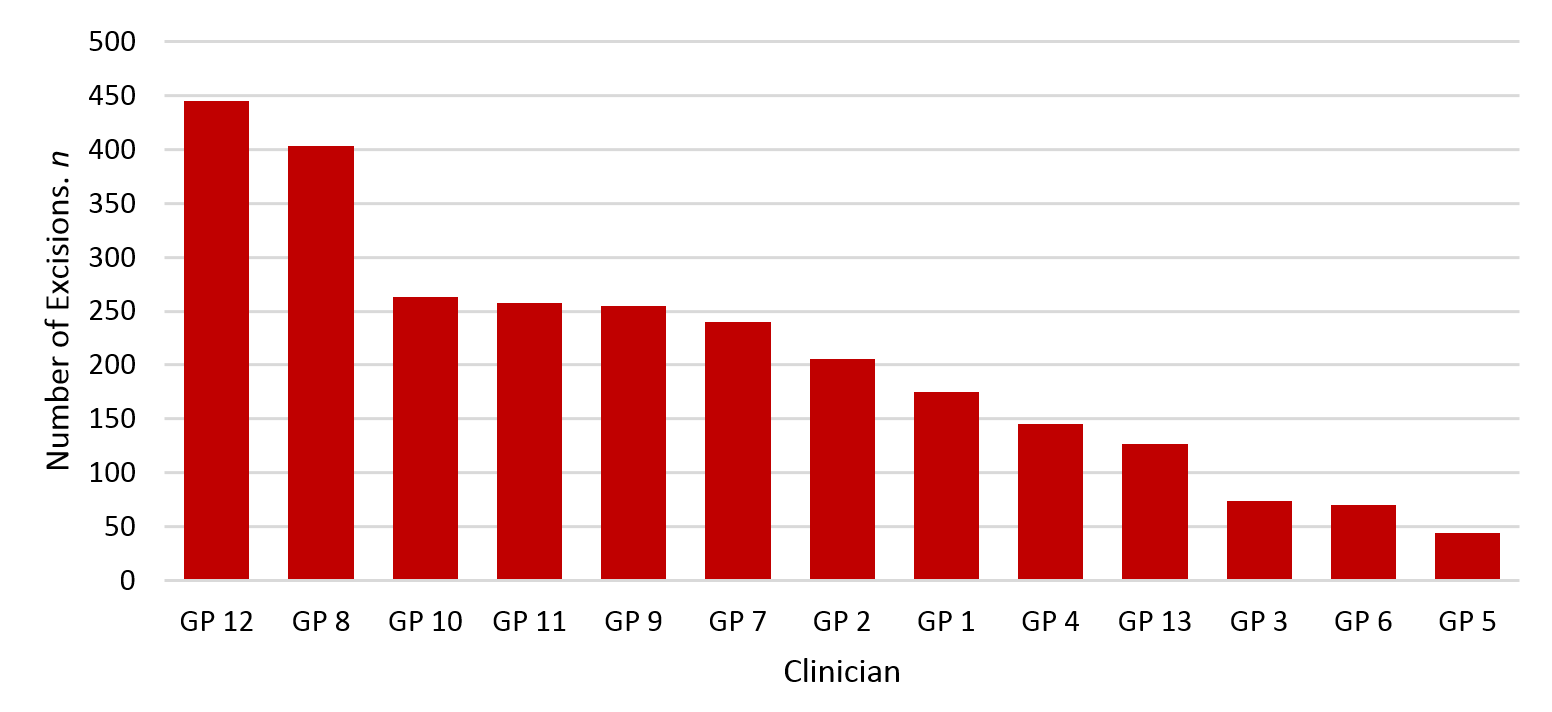
During the period 1 January 2016 to 31 December 2016, the 13 WDHB GPSIs excised a total of 2,705 lesions on 1,643 patients.
Figure 1: Total number of excisions by clinician.
Types of lesions removed
Basal cell carcinomas (all types) were the most frequently excised lesion, making up a total of 1,173 (42.3%) lesions. Please refer to Table 1 for a full breakdown.
Table 1:
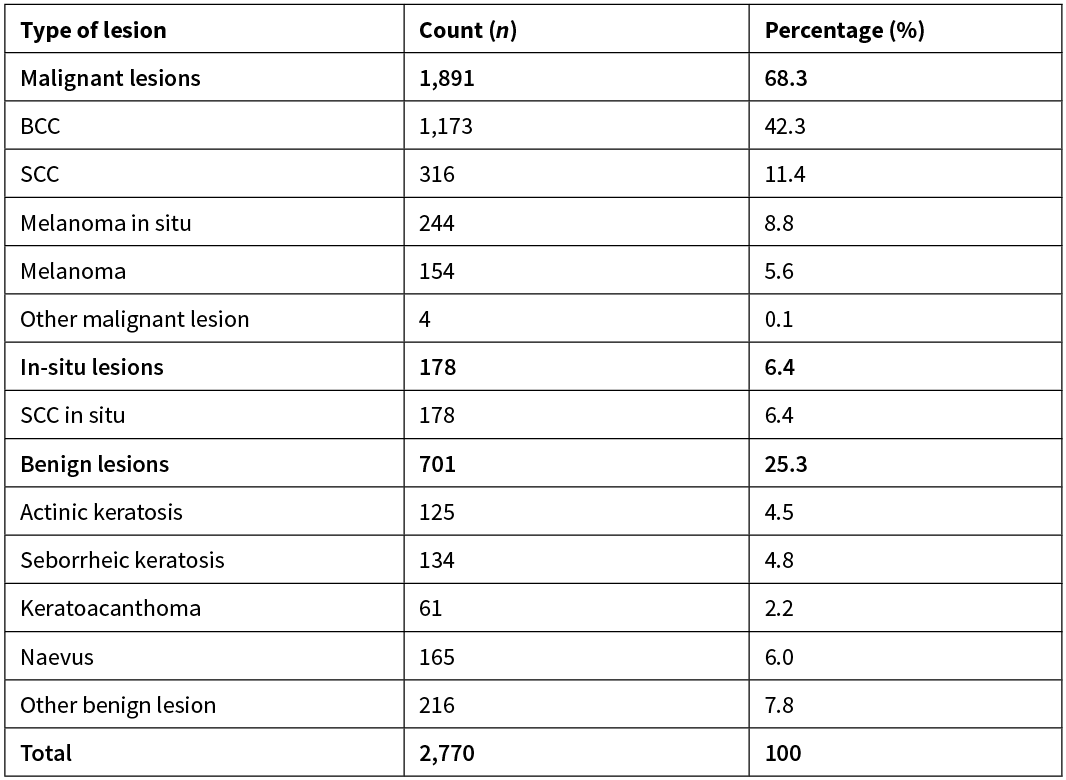
Total number of histological lesions exceeds number of excisions performed by GP surgeons because 63 excisions contained two histologically discrete lesions and one excision contained three histologically discrete lesions; margins were analysed for each discrete lesion by the same criteria as other excisions.
The four ‘other malignant lesion’ category included: one small lymphocytic lymphoma secondary to chronic lymphocytic leukaemia, one follicle centre lymphoma, one Merkel cell carcinoma and one pleiomorphic dermal sarcoma. The 216 ‘other benign lesions’ were primarily: haemangioma, dermatofibromas, lichenoid keratoses and epidermal cysts.
Location of excisions
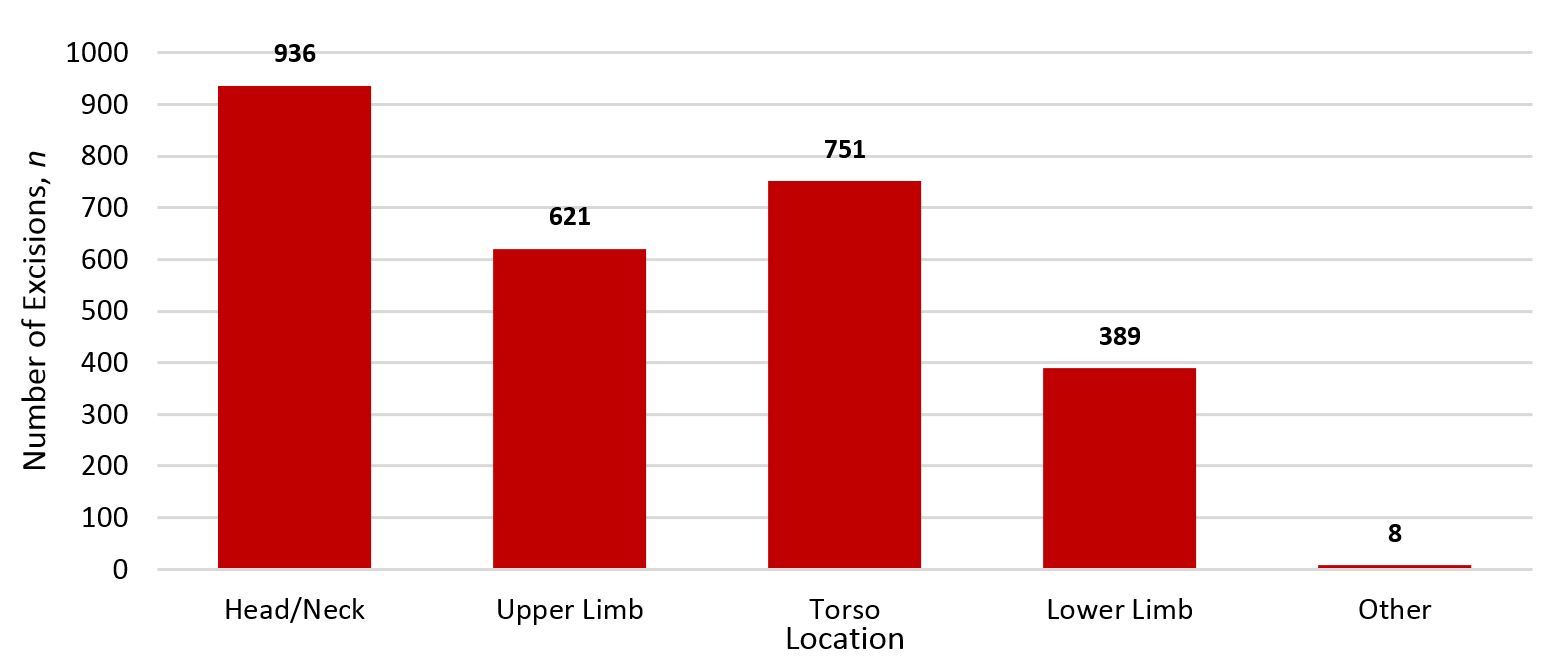
Lesions on the head and neck were the most commonly excised location with 936 (34.6%) excisions.
Figure 2: Location of lesions removed.
Positive margin rate of malignant lesions
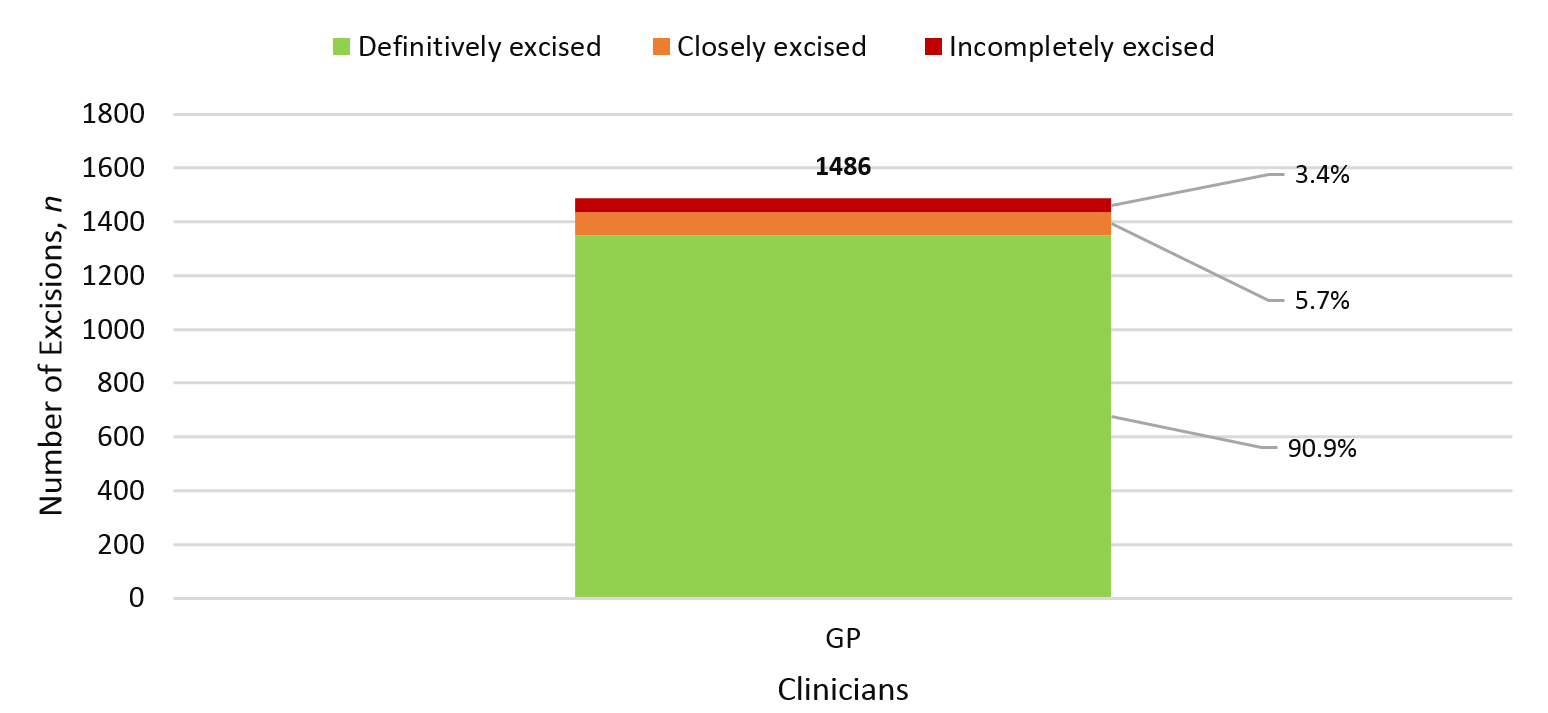
During the one-year period, WDHB GPSIs removed 1,887 malignant lesions (69.8% of all excisions), of which 1,486 lesions were NMSCs. Among NMSCs, an incomplete surgical margin was observed in 51 (3.4%) excisions and 84 cases (5.7%) had margins that were clear but closely excised.
Figure 3: Positive margin rate of NMSC.
The effect of lesion location was analysed; showing the head/neck region to have the highest positive margin rate of 5.3% across the excision of 1,486 lesions.
Figure 4: Positive margin rate of NMSC by location.
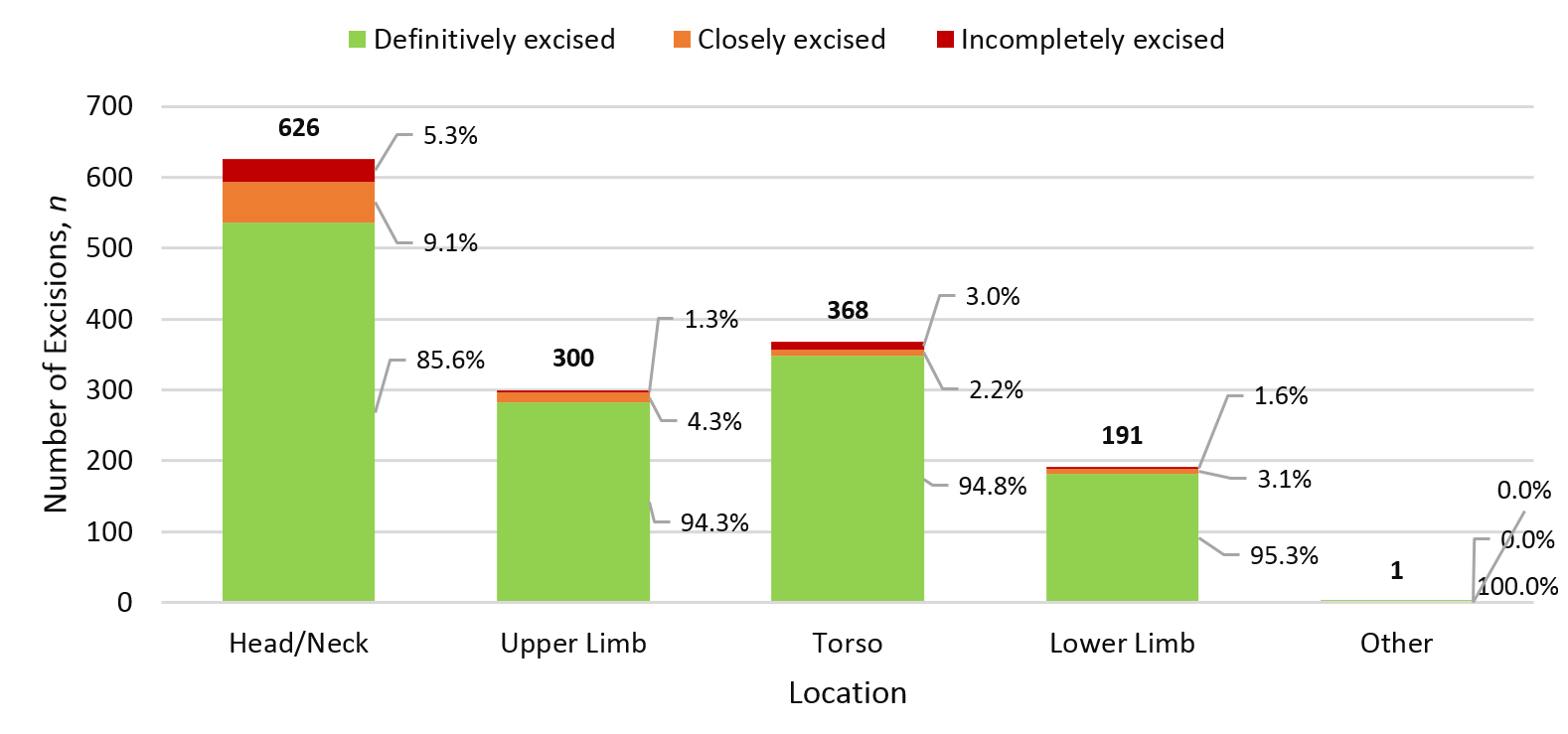
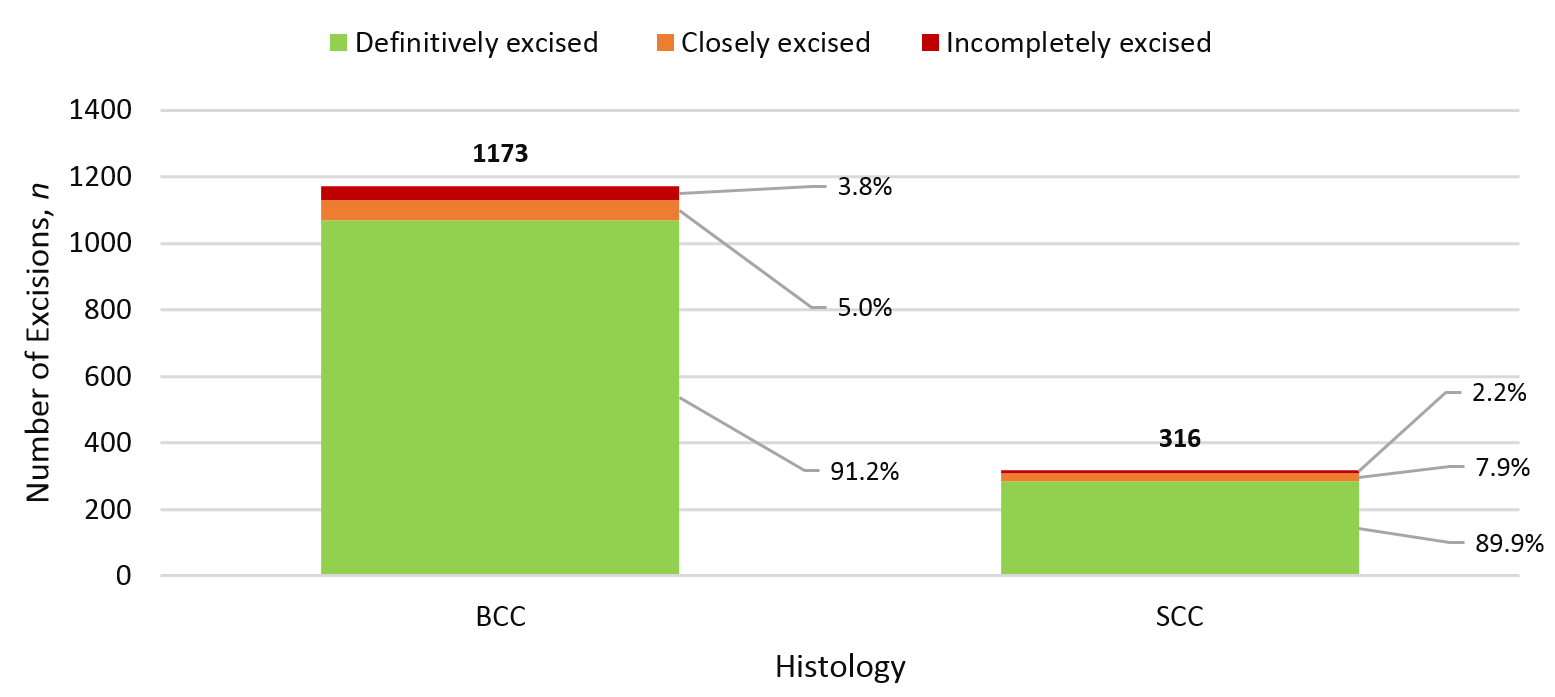
Figure 5: Positive margin rate by histology.
Re-excision rates
There were a recorded 46 lesions which required re-excision due to inadequate margins. This equates to a re-excision rate of 1.7%.
Infection rates
There were a total of 294 (10.9%) cases of infection among all 13 GPSIs within the one-year period. The average and median time to evidence of an infection (ie, prescription of antibiotics) were 11.7 days and 10 days respectively. There are no clinic reports or follow up letters available for the WDHB GPSI clinics and therefore we cannot determine if there were other complications but WDHB is unaware of any serious complications or complaints.
Time to treat
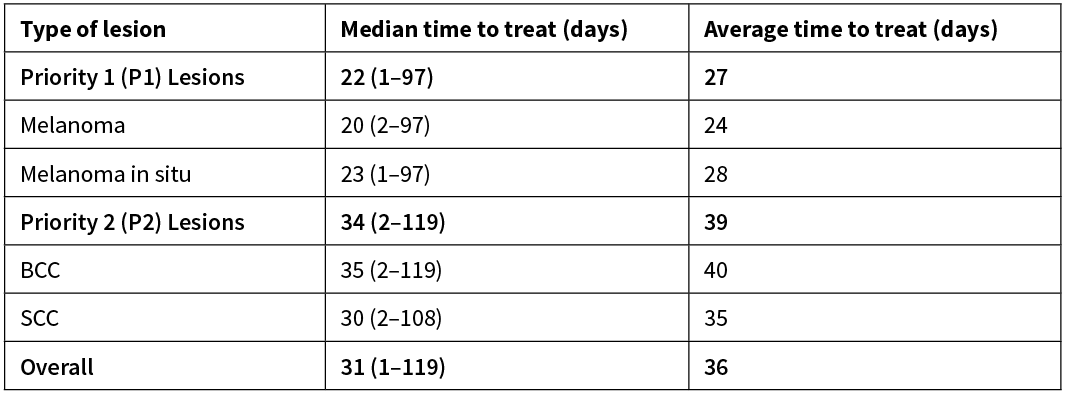
Across all excisions, median time to treat was 31 days and the average time to treat was 36 days. For Priority 1 (P1) lesions, median and average time to treat were 22 and 27 days respectively. For Priority 2 (P2) lesions, median and average time to treat were 34 and 39 days respectively. A further breakdown of time to treat is available in Table 2.
Table 2:
Discussion
In 2016, there were a total of 2,705 excisions performed by 13 GPSIs under the WDHB Skin Service. Of these lesions, 1,887 were malignant lesions making up 70% of all excisions. Across the excision of 1,486 NMSCs, the WDHB GPSIs achieved a clearance rate of 96.6%. With regards to infection, the GPSIs had 294 (10.9%) cases of infection over all 2,705 excisions within our study period, with an average time to antibiotic prescription of 11.7 days. Median time to treat was 31 days for all lesions and median time to treat was 22 days for Priority 1 (P1) lesions.
This study is one of the largest retrospective audits to date observing the performance of GPs in the management of cutaneous malignancies. This review is, to our knowledge, the first that investigates the performance of primary care doctors following specialist surgical training and will assist in validating the efficacy of such practitioners in the management of skin cancer.
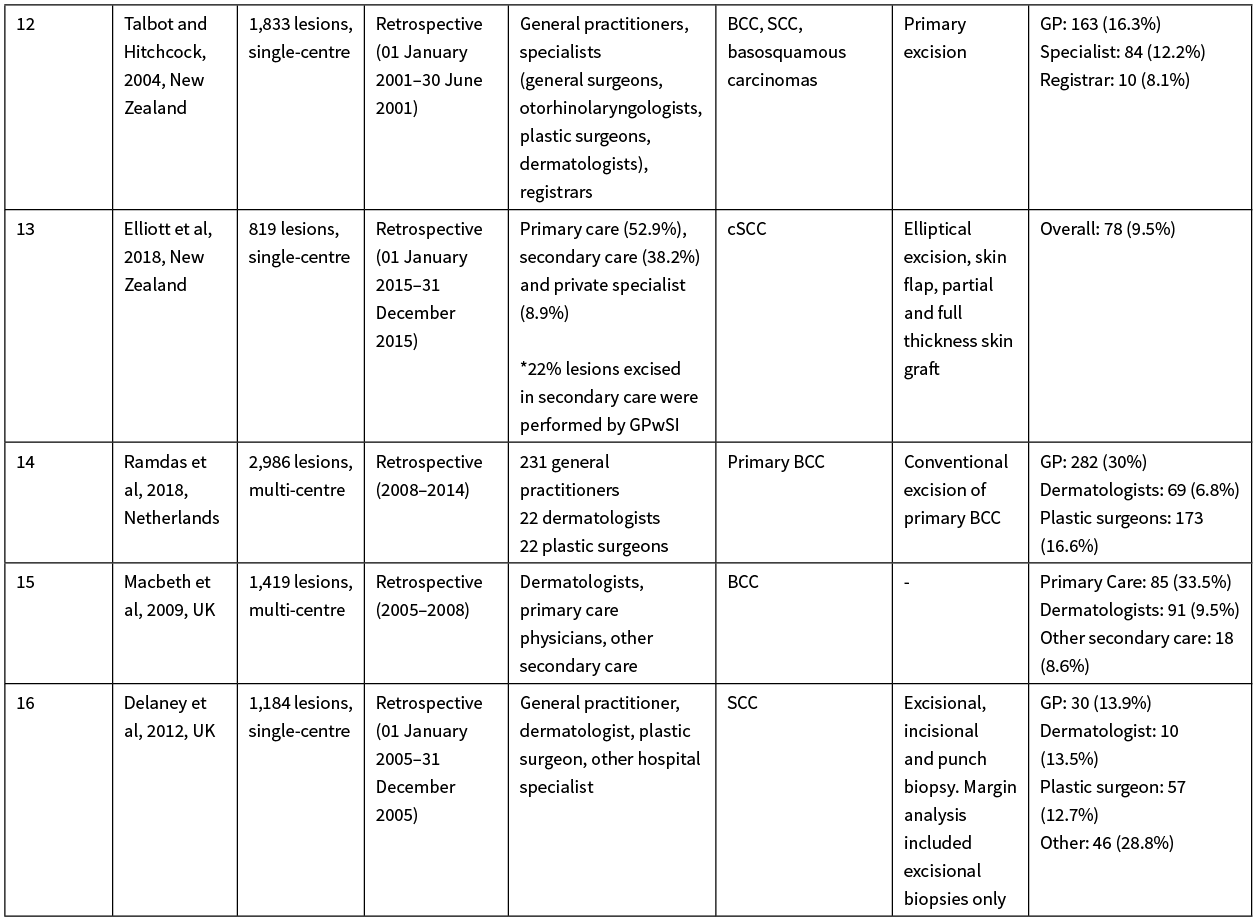
Historically, the performance of GPs in New Zealand excising cutaneous malignancies has been poorly documented. A study 20 years ago determined the NMSC excision positive margin rate among GPs in New Zealand to be 31%, while another study 14 years ago from the Bay of Plenty observed a GP-positive margin rate of 16%.11,12
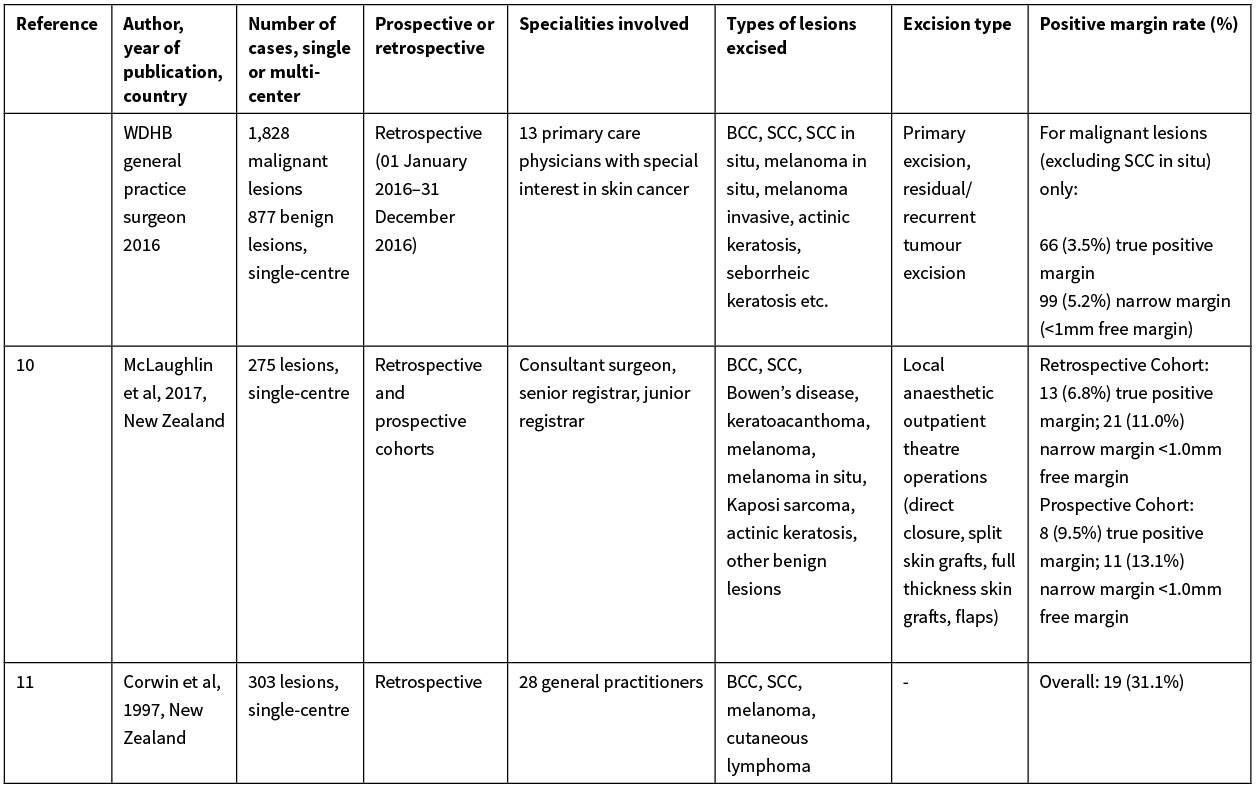
Consequently, we believe the best New Zealand standard to compare the WDHB GPSIs performance against is Counties Manukau District Health Board, Auckland, New Zealand’s plastic surgical unit data (McLaughlin et al 2017); their paper described consultant surgeons and surgical registrars achieving a positive margin rate of 6.8–9.5% over 275 local anaesthetic procedures—for benign and malignant lesions (BCC, SCC, Bowen’s disease, keratoacanthoma, melanoma, melanoma in situ, Kaposi sarcoma, actinic keratosis and other benign lesions).10 Additionally, Elliott et al (2018) observed a 9.5% positive margin rate among GPs in Northland, New Zealand for the treatment of cutaneous squamous cell carcinomas.13 Other published data originating from the UK and Netherlands describes variable GP NMSC excision rates, ranging from a 13.9% to 33.5% positive margin rate.14–16 Considering this data and the case mix of the WDHB GPSI, it is clear the WDHB GPSI’s 96.6% negative margin rate is an impressive feat; additionally, these studies provide a baseline context to the performance of New Zealand GPs excising malignant skin lesions in the primary care setting over the past two decades. Our study demonstrates the WDHB GPSI scheme has achieved a significantly improved negative margin rate for malignant skin excisions in comparison both locally and internationally. Please refer to the Appendix for a detailed breakdown and comparison of the aforementioned publications.
A review of literature around existing primary care skin cancer protocols in New Zealand reveals a programme in CDHB that is a relevant comparator to the WDHB GPSI scheme.17 The CDHB approach is a plastic surgeon-led see and treat clinic-based programme with the aim of increasing overall cutaneous surgical skills among all GPs in the region through six half-day sessions, compared to the WDHB scheme, which focuses on upskilling a focused group of GPs over a six-month training period in skin cancer surgery. At the time of the publication, the CDHB program had been active for six years and their complete excision rate was 92% and 94% across malignant and all lesions respectively, however they did not include a definition for ‘complete excision’ and a definition for ‘malignant lesion’. The WDHB GPSI achieved a complete excision rate/clear margin rate of 96.6% across all NMSC excisions. McGeoch et al also displayed their benign to malignant ratio in the form of a graph, however no data table was available to reference absolute figures; based on our interpretation of their graph, it appeared that roughly 54% of all excised lesions were malignant (estimated 1,300 malignant and total 2,400 lesions excised in 2013–2014). The data from WDHB GPSI scheme identified that 74.7% of lesions excised were malignant when including SCC in situ, and 68.3% of lesions excised were malignant if excluding SCC in situ.
When analysing by location, we identified that the head and neck (n=626) was the most poorly excised region with a positive margin rate of 5.3% and close margins observed in 9.1% of excisions; in comparison the positive margin rate for the torso and limbs ranged from 1.3–3.0%. This increased positive margin rate likely reflects the difficulty of excising lesions on the head and neck and the proximity of neighbouring structures, which may limit adequate margins during excisions.
The calculated re-excision rate is 1.7% (n=46). There is a small difference in re-excision rate between the five highest volume GPSI and five lowest volume GPSI; with a re-excision rate of 1.6% (26 of 1,598 lesions) and 2.7% (12 of 448 lesions) respectively. However, it should be noted that this re-excision rate reflects the number of cases re-excised by a GPSI and does not include cases that were subsequently referred to hospital for surgery due to inadequate margins. Based on protocol, all incomplete excisions and close margins were re-evaluated and a decision was made whether or not to re-excise by a specialist surgeon. Therefore, the closest estimate of our true re-excision rate is the positive margin rate of 3.4%. It should be noted that in select cases, patients may refuse surgery or a decision was made to observe/withhold surgery; therefore, the positive margin rate may overestimate our re-excision rate.
Literature review of post-operative infection among GPs reveals limited data, with one Australian study finding an 8.7% infection rate for minor skin excisions at all body sites (including melanoma, BCC, SCC and other benign lesions).18 We identified a greater infection rate (11.9%) among WDHB GPSI, however our measures are likely to be an overestimation considering the criteria for an infection may include non-excision related infections and variable antibiotic prescription practices. A proxy measure for infection rate was used as no clinical information on the wounds were available to review in conjunction with antibiotic prescribing information. Aside from returning to theatre for positive surgical margins, we were unable to determine if any excisions required a revision due to post-operative infection and/or other complications due to lack of clinical follow-up information in the GP rooms.
Patient selection is an important consideration for GP management versus specialist management of skin cancers. For the WDHB Skin Service, lesions are triaged by consultant surgeons at WDHB through an e-referral system with attached photos of lesions from general practitioners. The consultant surgeon will decide if dermatoscopy imaging is required for the pigmented lesions, if the lesion needs to be excised at hospital by a surgeon, or if the lesion can be excised by a GPSI. If the latter applies, an e-referral is sent to one of the GPSI doctors and the patient receives a letter notifying them of this.
The outstanding key performance indicators (KPIs) of our skin cancer program indicated by this study are the result of GP training and close mentor supervision. WDHB GPSIs have completed College-accredited postgraduate skin cancer surgery and dermatoscopy courses and spend six months of in-house training with two specialist cutaneous oncology surgeons. GPSI operated in a variety of settings including hospital operating theatres as well as procedural rooms in their own practices; upon completing their training, GPSI operate nearly full-time in their own facilities. All GP facilities are visited, logbooks are audited every six months and signed off as acceptable best practice (including sterilisation, lighting, electrocautery, facility, AED etc) to monitor KPIs. GPSIs are credentialed for simple excisions and more complex grafts and flaps depending on their skill level.
In 2018 alone, 229,867 keratinocytic cancers were estimated to be diagnosed in New Zealand and, considering New Zealand’s rapidly growing and ageing population, it is reasonable to assume the number of NMSCs treated will increase annually in the next decade.3,19 Most recent data from the New Zealand Cancer Registry recorded an age-standardised melanoma registration rate of 35.1 per 100,000 in 2017.20 In addition, although the invasive melanoma incidence in New Zealand appears to be plateauing for the last two decades, WDHB specific age-standardised melanoma rates increased by 14% from 44.2 per 100,000 in 1995–1999 to 50.2 per 100,000 in 2000–2004.8,21 In spite of this data, these melanoma incidence rates are in fact likely to be underestimations of the true incidence of melanoma within at-risk populations, as Māori were estimated to have a melanoma incidence of 2.3 per 100,000 in 2000–2004.22 Compounded with changes to private healthcare policies and veteran affairs limiting skin cancer claims, more patients are being driven into the public sector inflating the problem of managing enormous skin cancer volumes. Consequently, the funding bodies in New Zealand must ensure adequate financial support and planning for the management of skin cancer. This requires a multi-disciplinary collaborative approach to skin cancer management, including dermatologists, surgeons and general practitioners.
WDHB GP surgeons achieved a negative margin rate of 96.6% in 2016 for the excision of NMSC. This is in accordance with international guidelines that expect a 95% clearance rate for NMSC excision.23–25 GP surgeons reduce the length of time patients wait before having their lesions assessed and/or excised; median time to treat for Priority 1 lesions is 22 days and overall median time to treat is 31 days. FY2017 WDHB Skin Service data demonstrates workload and cost per case figures that showed an 88% cost reduction per case to WDHB over a large caseload and therefore supports the involvement of GPSI in skin cancer management. It is important to consider that the cases managed at the secondary/tertiary centres are more complex and resource intensive; however, this does not discredit the cost-effectiveness of GPSI at managing large volumes of simple cutaneous malignancies. Overall, the WDHB GPSIs improve the efficiency of our skin cancer service and assist in the treatment of cutaneous malignancies in a timely and cost effective manner.
Our data supports the implementation of GPSIs as part of New Zealand’s skin cancer workforce, where they will be an integral part of the multi-disciplinary team managing cutaneous malignancies. The WDHB GPSI scheme has become a reliable, efficient and safe resource for the management of malignant skin lesions in our community with a negative margin rate well below acceptable international guideline standards.
Conclusion
This study validates the safe use of GP surgeons and shows their integral role in managing the enormous volume of skin cancer in New Zealand. This data would suggest that all district health boards in New Zealand should allocate resources to and utilise GPs in the management of skin cancer.
Appendix
See more related
Aim
Waitemata District Health Board has implemented a new approach to the management of skin cancers by triaging lesions to specialist-trained general practitioners (GPSI) with the aim of reducing patient wait times and treatment costs. The primary outcome was to determine positive margin rates for the GP surgeons, with secondary outcome being infection rates.
Methods
A retrospective audit was conducted on all excisions (n=2,705) performed between 1 January 2016 and 31 December 2016 by the 13 WDHB GPSIs. Electronic patient records were accessed to review data. Each lesion was classified into benign, in-situ (pre-malignant) and malignant categories. Surgical margins were analysed for non-melanotic skin cancers (NMSC) and determined as positive, close or negative. Infection rates determined by microbiology results and prescribing information and time to treat analyses were conducted.
Results
WDHB GPSIs performed 2,705 excisions, 1,887 (69.8%) of which were malignant lesions. Among the 1,486 NMSC excised, a positive surgical margin was observed in 51 (3.4%). There were 294 (10.9%) cases of infection in 2,705 excisions. Median time to treat was 31 days across all lesions. New Zealand papers from the last two decades estimate the NMSC positive margin rate among primary care physicians varies between 16–31%; most recent papers have published rates 6.8–9.5%.European publications describe positive margin rates ranging between 13.9–33.5%.
Conclusion
WDHB GPSIs performed 2,705 excisions, 1,887 (69.8%) of which were malignant lesions. Among the 1,486 NMSC excised, a positive surgical margin was observed in 51 (3.4%). There were 294 (10.9%) cases of infection in 2,705 excisions. Median time to treat was 31 days across all lesions. New Zealand papers from the last two decades estimate the NMSC positive margin rate among primary care physicians varies between 16–31%; most recent papers have published rates 6.8–9.5%.European publications describe positive margin rates ranging between 13.9–33.5%.
Authors
Daniel Wen, Medical Student, Faculty Medical and Health Sciences, University of Auckland, Auckland; Katherine Gale, Breast Oncoplastic Surgeon, Department of Cutaneous Oncology, North Shore Hospital, Waitemata District Health Board, Auckland; Richard Martin, Cutaneous Surgical Oncologist, Department of Cutaneous Oncology, North Shore Hospital, Waitemata District Health Board, Auckland.Correspondence
Daniel Wen, Faculty Medical and Health Sciences, University of Auckland, Auckland.Correspondence email
dwen536@aucklanduni.ac.nzCompeting interests
Daniel Wen, Katherine Gale, Richard CW Martin1. Freeman NR, Fairbrother GE, Rose RJ. Survey of skin cancer incidence in the Hamilton area. The New Zealand Medical Journal, 1982; 95(713):529–533.
2. O’Dea D. A Report to the Cancer Society. The Costs of Skin Cancer to New Zealand: Wellington School of Medicine, University of Otago. 2000.
3. Sneyd MJ, Gray A. Expected non melanoma skin (Keratinocytic) cancer incidence in New Zealand for 2018. Wellington: Health Promotion Agency. 2018
4. Pondicherry A, Martin R, Meredith I, et al. The burden of non-melanoma skin cancers in Auckland, New Zealand. Australasian Journal Of Dermatology, 2018; 59(3):210–213.
5. O’Dea D. The costs of skin cancer to New Zealand. The Cancer Society of New Zealand. 2009.
6. Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. British Journal Of Dermatology, 2012; 166(5):1069–1080.
7. Fransen M, Karahalios A, Sharma N, et al. Non-melanoma skin cancer in Australia. The Medical Journal Of Australia, 2012; 197(10):565–568.
8. Liang J, Robinson E, Martin R. Cutaneous melanoma in New Zealand: 2000-2004. ANZ Journal Of Surgery, 2010; 80(5):312–316.
9. Whiteman D, Green A, Olsen C. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. Journal of Investigative Dermatology, 2016; 136(6):1161–1171.
10. McLaughlin S, Kenealy J, Locke M. Effect of a See and Treat clinic on skin cancer treatment time. ANZ Journal of Surgery, 2017; 88(5):474–479.
11. Corwin P, Munn E, Nicholls D. A study of general practitioners’ skin surgery in Canterbury. The New Zealand Medical Journal, 1997; 110(1047):253–255.
12. Talbot S, Hitchcock B. Incomplete primary excision of cutaneous basal and squamous cell carcinoma in the Bay of Plenty. The New Zealand Medical Journal, 2004; 117(1192):U848.
13. Elliott BM, Douglass BR, McConnell D, et al. Incidence, demographics and surgical outcomes of cutaneous squamous cell carcinoma diagnosed in Northland, New Zealand. The New Zealand Medical Journal, 2018; 131(1475):61–68.
14. Ramdas K, van Lee C, Beck S, et al. Differences in Rate of Complete Excision of Basal Cell Carcinoma by Dermatologists, Plastic Surgeons and General Practitioners: A Large Cross-Sectional Study. Dermatology, 2018; 234(3–4):86–91.
15. Macbeth A, Torley D, Casie Chetty N, et al. Audit of incomplete excision rates of basal cell carcinoma: analysis of 1972 cases from four U.K. regions. British Journal Of Dermatology, 2009; 161(3):710–712.
16. Delaney E, Duckworth L, Thompson W, et al. Excising squamous cell carcinomas: comparing the performance of GPs, hospital skin specialists and other hospital specialists. Family Practice, 2012; 29(5):541–546.
17. McGeoch G, Sycamore M, Shand B, Simcock J. A regional programme to improve skin cancer management. Journal of Primary Health Care, 2015; 7(4):339.
18. Heal C, Buettner P, Drobetz H. Risk factors for surgical site infection after dermatological surgery. International Journal of Dermatology, 2012; 51(7):796–803.
19. MacPherson L. National Population Projections: 2016(base) – 2068. Statistics New Zealand. ISSN 1178-0584. 2016.
20. Selected cancers 2015, 2016, 2017. New Zealand Cancer Registry, Ministry of Health, New Zealand. 2019.
21. Martin R, Robinson E. Cutaneous melanoma in Caucasian New Zealanders: 1995-1999. ANZ Journal of Surgery, 2004; 74(4):233–237.
22. Hore T, Robinson E, Martin R. Malignant Melanoma Amongst Maori and New Zealand Europeans, 2000–2004. World Journal Of Surgery, 2010; 34(8):1788–1792.
23. Telfer N, Colver G, Morton C. Guidelines for the management of basal cell carcinoma. British Journal Of Dermatology, 2008; 159(1):35–48.
24. Motley R, Kersey P, Lawrence C. Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. British Journal Of Dermatology, 2002; 146(1):18–25.
25. Basal cell carcinoma, squamous cell carcinoma (and related lesions) - a guide to clinical management in Australia. Cancer Council Australia and Australian Cancer Network, Sydney. 2008.
