ARTICLE
Vol. 133 No. 1511 |
Incidence of venous thromboembolism after total hip, total knee and hip fracture surgery at Waitemata District Health Board following a peer-reviewed audit
Venous thromboembolism (VTE) remains a significant cause of potentially preventable morbidity and mortality following total hip arthroplasty (THA), total knee arthroplasty (TKA) and hip fracture surgery.
Full article available to subscribers
Venous thromboembolism (VTE) remains a significant cause of potentially preventable morbidity and mortality following total hip arthroplasty (THA), total knee arthroplasty (TKA) and hip fracture surgery. The decision for chemoprophylaxis is balanced with the consequences of an increased risk of bleeding. Additional measures, such as mechanical prophylaxis also reduce the risk of post-operative VTEs.1 Current multimodal techniques involve a multidisciplinary approach towards reducing total VTE load.
The rates of VTE without chemical or mechanical prophylaxis are high; DVTs develop in 39–74% of patients undergoing THA,2 with the majority of these being asymptomatic. Half of all proximal DVTs, that is, those occurring in the popliteal vein or more proximally, are associated with PE,3 and up to half of patients with a proximal DVT will develop post-thrombotic syndrome.4 Fatal PEs occur after 0.4% of hip and knee arthroplasties in the absence of prophylaxis.5 Age and medical comorbidities, including a history of thrombosis, previous and/or family history of VTE, congestive heart failure, varicose veins, obesity and hypertension, add to the risk of VTE .6 This risk is estimated at 1–3% a year.7
The American Association of Orthopaedic Surgeons (AAOS),8 American College of Chest Physicians,9 National Institute for Health and Care Excellence (NICE)10 and The New Zealand National Policy Framework: VTE Prevention11 have guidelines promoting the routine use of VTE thromboprophylaxis, although the chemoprophylactic agent of choice remains debatable. Guidelines recommend using one of several agents including heparin products, vitamin K antagonists, antiplatelet drugs and novel oral anticoagulant agents such as rivaroxaban, with the choice of agent left to the clinician. Mechanical prophylaxis such as intermittent compression devices and graduated stockings are now routinely used in combination with chemoprophylaxis.12
International literature suggests that the incidence of symptomatic VTE following lower limb arthroplasty or hip fracture surgery in patients taking prophylaxis ranges from 1.0%13 to 2.7%.14 Although most DVTs and PEs occur within the first 10 days of operation,15 due to shorter average lengths of stay, most events occur after discharge from hospital.16
This study examines the incidence of DVT and PE in patients undergoing hip arthroplasty, knee arthroplasty and hip fracture surgery at Waitemata DHB between 1 January 2013 and 31 December 2016. This audit is a follow-up to an audit performed over the time period 2006–2010 in our institutions, as previously published.15
Materials and methods
In 2004, a VTE database was established at Waitemata DHB by consultant haematologists to collect data on all VTE events at time of occurrence covering the entire DHB population, which now totals over 600,000 people. This is linked to the Waitemata DHB electronic database, which includes all inpatient data and was used for data collection and analysis.
We included all patients with radiologically diagnosed symptomatic DVT and/or PE occurring within three months of lower-limb arthroplasty and hip fracture surgery between 1 January 2013 and 31 December 2016. Only symptomatic patients are scanned, as Waitemata DHB does not routinely screen asymptomatic postoperative patients. Waitemata DHB uses whole-limb ultrasound to diagnose DVTs, and computed tomography pulmonary angiography (CTPA) or V/Q scan to diagnose PEs—with some, but not all PE diagnoses being followed with subsequent lower-limb ultrasound. Lower-limb DVT included distal, proximal and bilateral DVT. As virtually all VTE events within Waitemata DHB are referred to haematology thrombosis services, this would capture nearly all inpatient and outpatient VTEs occurring.
We excluded portal vein thromboses, upper limb thromboses, subsegmental PE and all VTEs following surgery at other institutions in order to provide comparable data to 2006–2010.15 In addition, all VTE event data were reviewed to ensure there were no duplicate patient entries in the database. The focus on THA, TKA and hip fracture surgery enabled comparison with our earlier audit performed at Waitemata DHB.15 Chemoprophylaxis data was extracted from the Pyxis® automated drug dispensing cabinet (Becton Dickinson, New Jersey, US).
Statistical analysis was performed using SAS version 9.4. DVT and PE rates were compared between procedure groups, and between the study period (2013–2016) and a previous audit period at Waitemata DHB (2006–2010).15 DVT and PE outcomes were analysed using logistic regression modelling and included procedure group in the model. Analyses were adjusted for sex, age and American Society of Anaesthesiologists’ (ASA) score.
Results
There were 6,379 THA, TKA and hip fracture surgical procedures performed at Waitemata DHB between 2013 and 2016. Of the patients, 3,698 were female (58%) and 2,681 male (42%), with average ages of 73.8 and 72.4 years respectively. There were fewer males than females in the hip fracture group (32.3%) compared to THA (45.2%) and TKA (45.6%). There was no significant difference in age between men and women. Patients in the hip fracture group were on average a decade older (80.7) than the knee arthroplasty (69.2) or hip arthroplasty (69.8) groups.
A total of 135 VTEs were identified as occurring within three months of surgery. These included 98 lower-limb DVTs and 37 PEs (see Table 1). Most DVTs were confined to the distal veins (71.4%). Proximal DVTs occurred more frequently after THA and hip fracture surgery (47.1% and 41.5% of all DVTs respectively) than after TKA (7.5% of all DVTs).
Table 1: Comparison of VTE rates after type of surgery 2013–2016.
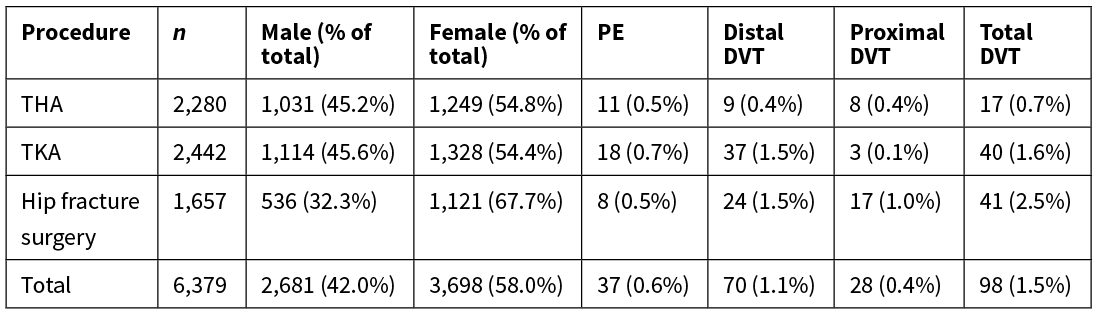
THA, total hip arthroplasty; TKA, total knee arthroplasty; n, number; DVT, deep vein thrombosis; PE, pulmonary embolism.
The overall DVT rate in this audit was 1.5%, a statistically significant reduction from 2.3% in 2006–201015 (OR 0.68, 95% CI 0.51–0.89; p=0.006) when adjusted for sex, age and ASA score (see Table 2).
The reduction in DVT rate was significant for THA (OR 0.49, 95% CI 0.26–0.92; p=0.025) and TKA (OR 0.44, 95% CI 0.29–0.67; p=0.001). The increase in DVT rate after hip fracture surgery from 2.0% to 2.5% was not statistically significant (see Table 2). There were significantly more DVTs occurring in both hip fracture (OR 2.94, 95% CI 1.53–5.65; p=0.001) and TKA patients (OR 2.13, 95% CI 1.20–3.78: p=0.01) compared to THA patients, calculated with adjusted logistical regression.
Table 2: Comparison of DVT rates between 2006–2010 and 2013–2016 by procedure.
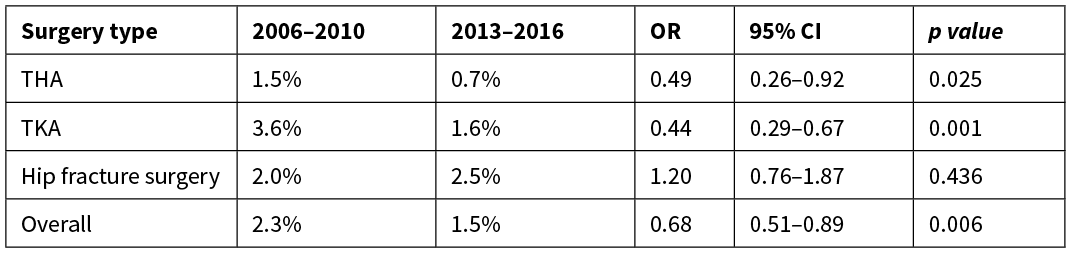
THA, total hip arthroplasty; TKA, total knee arthroplasty, OR, odds ratio; CI, confidence interval.
The overall PE rate in this audit was 0.6%. There was a statistically significant reduction in the adjusted rate of overall PE from 0.9% in 2006–2010 to 0.6% in 2013–2016 (OR 0.63, 95% CI 0.40–0.98: p=0.041), although the procedure-specific rates for THA, TKA and hip fracture surgery did not reach statistical significance at the p<0.05 level (see Table 3).
Table 3: Comparison of PE rates between the 2006–2010 and 2013–2016 periods by procedure.

THA, total hip arthroplasty; TKA, total knee arthroplasty, OR, odds ratio; CI, confidence interval.
DVT rates following hip fracture surgery were higher than DVT rates following THA or TKA, but there were no differences in PE rates between the three surgical groups.
The chemoprophylaxis data available were limited to a binary outcome of whether prophylactic agents were prescribed while patients were in hospital, and did not allow analysis of specific regimens, doses and duration of treatment. Overall, 92.6% of all patients (5,909 out of 6,379) and 92.6% of those who developed a post-operative VTE (125 out of 135) received inpatient chemoprophylaxis. This included 94.7% of THA patients, 86.7% of TKA patients and 94.7% of hip fracture surgery patients, with stable year-to-year trends across 2013 to 2016. Patients receiving more than one agent were included in each drug category, so were counted more than once overall. The agent used was most commonly LMWH, then aspirin, with smaller numbers of patients receiving direct oral anticoagulants, warfarin or unfractioned heparin. LMWH was prescribed in 60.7% of hip arthroplasty, 50.9% of knee arthroplasty and 57.0% of hip/femur fracture surgeries. Aspirin was used in 41.9% of THAs, 53.0% of TKAs and 49.5% of hip fracture surgeries. In total, 470 patients (7.6%) received no chemoprophylaxis, and 10 of these went on to develop a VTE (2.1%), which is the same VTE rate as the total surgical sample, and those who did not receive chemoprophylaxis. Case numbers are too small to allow any further analyses. In comparing the use of LMWH and aspirin in patients with VTE, LMWH use increased from 35% to 73%, while aspirin use slightly decreased from 59% to 57% from 2006–2010 to 2013–2016.
One patient died within three months of surgery, giving an all-cause mortality of 0.74%—compared with three patients and mortality rate 1.8% in the previous audit.15 Unfortunately, data are unavailable regarding the cause of death; however, this occurred after discharge from hospital following surgery.
Discussion
VTE rates following hip and knee surgery vary over time and between different institutions. Published rates range from 2.1–2.8%14,16 in older studies, to 0.64–1.2% in more contemporary research.13,17,18 Confounding variables occurring between institutions, population demographics and changes in practice can make comparisons difficult.
There was a significant fall in VTE rates between audits, with reductions in DVT from 2.3% to 1.5% and PE from 0.9% to 0.6%. The authors of the 2006–2010 audit15 concluded that their VTE rate was higher than that found in literature at the time, attributed to suboptimal chemical and mechanical prophylaxis. Direct comparison of thromboprophylaxis rates for all surgical patients is not possible as the earlier audit only presented this data for VTE cases. However, in this subgroup we note that in 2006–2010, 14% of postoperative VTE patients had received no chemoprophylaxis, compared to 7.4% in the current audit. This limited data may suggest increased rates of chemoprophylaxis over time.
This audit included a variety of surgical procedures, performed for different indications in a heterogeneous population. This heterogeneity can lead to a variation in VTE rate between types of procedure or indication for surgery. Bjørnarå et al found comparable rates of DVTs after hip fracture surgery to that of TKA and THA in the same era as our initial audit.14 In comparison, our current adjusted DVT rates were higher in the acute hip fracture surgery group compared to the elective THA and TKA groups. Compared to the previous audit, the current hip fracture DVT rate did not decrease and remained above 2%. However, DVT rates in the elective THA and TKA groups fell (p=0.025 and p=0.001 respectively).
The DVT rates include both proximal and distal DVT, and the relative proportion of these varied within procedure groups with higher proportions of distal DVTs after TKA compared to THA. Compared to the 2006–2010 audit,15 there was a reduction in the overall rate of distal DVTs for THA patients (0.9% to 0.4%), but a relatively stable rate of proximal DVTs (0.3% to 0.4%). This had the effect of reducing the relative proportion of distal DVTs following THA between audits (from 77.8% to 52.9%). In contrast, in the TKA group, it was the rate of proximal DVTs that fell between audits (from 0.6% to 0.1%), resulting in an increase in the relative proportion of distal DVTs (from 86% to 92.5%). This is also consistent with the established association of TKA with more distal DVTs.19 In the acute hip fracture surgery group, the proportion of proximal to distal DVT did not change between the two audits. With the three groups pooled, overall proximal DVT rate declined from 0.6% to 0.4% between the current period and 2006–2010.15 PE rates declined across all three groups in the subsequent audit when pooled (0.9% to 0.6% p=0.041) but not as individual treatment groups, likely due to the small number of these events occurring. Given that not all PE patients receive subsequent ultrasound to assess clot burden (but some do), we acknowledge the possibility that some pulmonary emboli may not be thrombotic in origin but may be from other sources such as bone or debris produced perioperatively.
The reasons for the unchanged DVT rate in hip fracture patients are likely multifactorial, such as older age, poor nutritional and hydration status pre-operatively, slower post-operative mobilisation and rehabilitation, poor pre-operative function and mobility, and delays in rehabilitation due to medical issues and investigations. Additionally, despite reductions in time to theatre as discussed below, hip fracture patients remain preoperatively immobilised for longer periods than elective THA and TKA patients. This group had significantly longer inpatient stays, with an average length of stay of 21 versus 4 days. We did not have sufficient data to include these factors in multivariate analysis. The appointment of an orthogeriatrician at the start of 2018, with a dedicated focus on the pre-operative and post-operative management of neck of femur fracture patients will medically optimise these patients and may help reduce their rates of VTE.
Considerable changes have occurred at Waitemata DHB between the time periods of the two audits, which we believe may have contributed to reductions in VTE rates in hip and knee arthroplasty patients. They are improved collaboration, enhanced recovery after surgery (ERAS) and VTE risk assessment.
Clinical leads have been set up in the orthopaedic surgery and haematology departments to facilitate liaison and provide feedback, along with input and analysis from a public health physician. Case study evaluation occurs between the disciplines on a regular basis, with an increase in dialogue between surgeons and physicians regarding more complex individual cases. There is heightened awareness of the implications of therapeutic anti-coagulation of a proven VTE soon after surgery, with haematology clinical nurse specialists providing a consistent point of contact for the investigation and management of these patients. Regular discussion of all proven VTE cases and their subsequent management occurs within the orthopaedic department every three months, and regular VTE meetings have been scheduled into standard teaching commitments to provide emphasis on the importance of VTE prophylaxis to junior staff.
ERAS protocols21 are a bundle of evidence-based multimodal interventions aimed at improving post-operative recovery and reducing complications by aggregating marginal gains in care before, during and after surgery. ERAS protocols were introduced during the current audit. Adherence to these protocols was easier in THA and TKA patients, taking longer to implement for hip fracture surgery and for this group, only being in effect towards the end of this audit. Hip fracture protocols focused particularly on reducing time to surgery, improving peri-operative analgesia enabling earlier postoperative mobilisation (within 24 hours), and cohorting patients in dedicated wards. Other data from Waitemata DHB indicates that there has been a reduction in average hours to theatre for hip fracture patients from 39 hours in 2010 to 29 hours in 2016, a decrease in the median hours to mobilisation from 44 to 24 hours, and an associated reduction in crude 30-day mortality rate from 10.2% to 5.7%.22
Although ERAS protocols do not make specific recommendations regarding the use of chemoprophylaxis, other ERAS measures such as early post-operative mobilisation and early hospital discharge have been linked to reduced VTE rates after orthopaedic surgery.23 A recent cohort study showed a significant reduction in median length of stay after elective hip and knee arthroplasty after implementing ERAS protocols without an increase in complications.24 However, the link between ERAS and reduced VTE rates is controversial, with other authors reporting no link between ERAS protocols and VTE rates after arthroplasty despite reductions in overall death rates, rates of myocardial infarctions and strokes.21,25 The National Policy Framework “DVT/PE Prevention in Hospitalised Patients in New Zealand guidelines”11 recommends the routine use of chemoprophylaxis after hip arthroplasty, knee arthroplasty and hip fracture surgery. The rate of chemoprophylaxis use was 92.6% and was identical between both the denominator and the numerator groups for diagnosed VTE. This is an improvement from the 86% numerator rate in 2006–2010.15 No VTE risk stratification tools were used in Waitemata DHB during the audit period, although VTE risk stratification is done for all orthopaedic patients since 2018. Chemoprophylactic agents were more widely prescribed after THA and hip fracture surgery (both 94.7%) than in TKA (86.7%), with LMWH and aspirin the most commonly used agents.
Some studies show aspirin to be as efficacious for VTE prophylaxis after hip and knee arthroplasty as LMWH,13,26–29 but this remains contentious.9 The AAOS and ACCP have now endorsed aspirin as a viable chemoprophylaxis agent in low-risk groups undergoing arthroplasty.30 The use of novel anticoagulants such as rivaroxaban and dabigatran is increasing, both within the general population and as prophylaxis following surgery, particularly after TKA. This, along with an increased use of aspirin, appears to coincide with less use of LMWH in TKA after 2013 in our audit.
Warfarin, dabigatran and rivaroxaban were utilised more in acute hip fracture patients compared to THA and TKA patients in this audit. This was most likely a reflection of these patients being more comorbid and being on these medications prior to admission and surgery.
The strengths of this study were that there were no exclusions of any patient and the numbers audited are large for a public hospital in New Zealand. The limitations with this audit relate mainly to data collection. In addition, there may be additional VTEs in this post-operative population that cause death prior to radiographical diagnosis. By their nature, these would not be included in our data. The chemoprophylaxis data was collated from PYXIS. It collates the rate of inpatient prescription, but not the timing (first or subsequent doses), the dosage or the duration of prophylaxis. There are no data about the patients that were not given chemoprophylaxis, either the reasons for this or alternative medications or measures taken.
Patients who received more than one agent were included in each drug category, so were counted more than once. We are unable to say how many dual therapy patients were in each group. Data are unavailable regarding the type and duration of mechanical prophylaxis. A mixture of calf compression devices, foot pumps and TED stockings were used, both intra-operatively and post-operatively. Documentation of mechanical prophylaxis is poor compared to chemoprophylaxis. Electronic prescribing was in place at Waitemata DHB by the end of 2016, so future audits can collect accurate and detailed data regarding inpatient dosing and timing of VTE chemoprophylaxis and TED stockings. Similarly, electronic discharge summaries and electronic records of community dispensing can record chemoprophylaxis after discharge.
Conclusion
There has been a significant reduction in post-operative VTE rates at our institution following hip and knee arthroplasty and hip fracture surgery, from 3.2% (2006–2010)15 to 2.1% (2013–2016). We believe that this has been contributed to by a multidisciplinary approach with improved collaboration, the introduction of ERAS protocols and VTE risk assessment tools, and an increase in chemo-prophylaxis rates.
See more related
Aim
The incidence of venous thromboembolism (VTE) following arthroplasty and hip fracture surgery remains an important metric for quality and financial reasons. An audit at our institution between 2006–2010 showed a higher VTE rate than international data did at the time. This study aims to determine rates of DVT and PE in patients undergoing hip and knee arthroplasty and hip fracture surgery at Waitemata District Health Board (Waitemata DHB) between 1 January 2013 and 31 December 2016.
Methods
This study is a retrospective review of all VTE within three months of elective hip or knee replacement or hip fracture surgery. Data were identified for the period between 2013 and 2016 from Waitemata DHB patient databases, including a dedicated VTE database.
Results
The current rates of deep vein thrombosis (DVT) and pulmonary embolism (PE) at our institution following hip or knee arthroplasty or hip fracture surgery are 1.5% and 0.6% respectively, a lower rate than 2.3% and 0.9% respectively in 2006–2010. DVTs were significantly more prevalent after hip fracture surgery than after elective hip or knee arthroplasty, and 71% of DVTs were confined to the distal veins. Of the patients undergoing surgery, 93% received post-operative chemoprophylaxis, mainly aspirin or low molecular-weight heparin (LMWH).
Conclusion
There has been a significant reduction in VTE rates following elective hip and knee joint replacement and hip fracture surgery between the time periods. This occurred over a period when Waitemata DHB introduced a multi-modal, interdisciplinary team approach to VTE prophylaxis utilising enhanced recovery after surgery (ERAS) pathways. These measures may therefore have contributed to the reduction in VTEs.
Authors
James S Millar, Orthopaedic Registrar, Orthopaedic Department, North Shore Hospital, Waitemata District Health Board, Auckland; Carlene MM Lawes, Public Health Physician, North Shore Hospital, Waitemata District Health Board, Auckland; Bill Farrington, Orthopaedic Surgeon, Department of Orthopaedic Surgery, North Shore Hospital, Waitemata District Health Board, Auckland; Penny Andrew, Director of the Institute for Innovation and Improvement, North Shore Hospital, Waitemata District Health Board, Auckland; Peter Misur, Orthopaedic Surgeon, Department of Orthopaedic Surgery, North Shore Hospital, Waitemata District Health Board, Auckland; Eileen Merriman, Consultant Haematologist/Lead Thrombosis Clinician, Department of Haematology, North Shore Hospital, Waitemata District Health Board, Auckland; Matt Walker, Clinical Director of Orthopaedics, Department of Orthopaedic Surgery, North Shore Hospital, Waitemata District Health Board, Auckland.Acknowledgements
Varsha Parag, Biostatistician, National Institute of Health Innovation, University of Auckland; Delwyn Armstrong, Head of Analytics, Waitemata District Health Board, Auckland; Monique Greene, Information Analyst, Waitemata District Health Board, Auckland.Correspondence
James S Millar, Orthopaedic Registrar, Orthopaedic Department, North Shore Hospital, Waitemata District Health Board, Auckland.Correspondence email
james.s.millar@gmail.comCompeting interests
Dr Farrington reports personal fees from Stryker, personal fees from LIMA Orthopaedics, outside the submitted work.1. Welfare M. NICE’s recommendations for thromboembolism are not evidence based. BMJ. 2011; 343:d6452. doi:10.1136/BMJ.D6452
2. Freedman KB, Brookenthal KR, Fitzgerald RH, et al. A meta-analysis of thromboembolic prophylaxis following elective total hip arthroplasty. J Bone Joint Surg Am. 2000; 82-A(7):929–938. http://www.ncbi.nlm.nih.gov/pubmed/10901307. Accessed October 11, 2016.
3. Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996; 93(12):2212–2245. http://www.ncbi.nlm.nih.gov/pubmed/8925592. Accessed May 23, 2018.
4. Baldwin MJ, Moore HM, Rudarakanchana N, et al. Post-thrombotic syndrome: a clinical review. J Thromb Haemost. 2013; 11(5):795–805. doi:10.1111/jth.12180
5. Warwick D. New concepts in orthopaedic thromboprophylaxis. J Bone Joint Surg Br. 2004; 86(6):788–792. http://www.ncbi.nlm.nih.gov/pubmed/15330015. Accessed September 17, 2016.
6. Leung K, Chiu K, Yan C, et al. Review Article: Venous thromboembolism after total joint replacement. J Orthop Surg. 2013; 21(3):351–360.
7. Tran HA, Chunilal SD, Harper PL, et al. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013; 198(4):198–199. http://www.ncbi.nlm.nih.gov/pubmed/23451962. Accessed May 23, 2018.
8. Jacobs JJ, Mont MA, Bozic KJ, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty. J Bone J Surg. 2012; 94(8):746–7
9. Colwell CW. The ACCP Guidelines for Thromboprophylaxis in Total Hip and Knee Arthroplasty. Orthopedics. 2009; 32(12/Supplement):67–73. doi:10.3928/01477447-20091103-51
10. NICE. National Institute for Health and Care Excellence (2018). Reducing Venous Othromboembolism: Orthopaedic Surgery.; 2018. http://pathways.nice.org.uk/pathways/venous-thromboembolism/reducing-venous-thromboembolism-risk-orthopaedic-surgery.pdf
11. Blumgart A, Merriman EG, Jackson S, et al. National Policy Framework: VTE Prevention in Adult Hospitalised Patients in NZ.; 2012.
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e278S-325S. doi:10.1378/chest.11-2404
13. Anderson DR, Dunbar M, Murnaghan J, et al. Aspirin or Rivaroxaban for VTE Prophylaxis after Hip or Knee Arthroplasty. N Engl J Med. 2018; 378(8):699–707. doi:10.1056/NEJMoa1712746
14. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006; 88(3):386–391. doi:10.1302/0301-620X.88B3.17207
15. Dixon J, Ahn E, Zhou L, et al. Venous thromboembolism rates in patients undergoing major hip and knee joint surgery at Waitemata District Health Board: a retrospective audit. Intern Med J. 2015; 45(4):416–422. doi:10.1111/imj.12702
16. Lapidus LJ, Ponzer S, Pettersson H, de Bri E. Symptomatic venous thromboembolism and mortality in orthopaedic surgery - an observational study of 45 968 consecutive procedures. BMC Musculoskelet Disord. 2013;14(1). doi:10.1186/1471-2474-14-177
17. Malhotra K, Marciniak JL, Bonczek SJ, Hunt N. Venous thromboembolism after lower limb arthroplasty: is chemical prophylaxis still needed? Eur J Orthop Surg Traumatol. 2016; 26(8):895–899. doi:10.1007/s00590-016-1820-9
18. Rolton D. The Rates of Thromboembolic Events in Patients Undergoing Elective Hip and Knee Arthroplasty before and One Year after the Introduction of the NICE Guidelines.; 2015. http://www.boa.ac.uk/wp-content/uploads/2015/06/The-rates-of-Thromboembolic-events-in-patients-undergoing-elective-hip-and-knee-arthroplasty-before-and-one-year-after-the-introduction-of-the-NICE-guidelines-References.pdf. Accessed August 26, 2018.
19. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126(3 Suppl):338S–400S. doi:10.1378/chest.126.3_suppl.338S
20. Cordell-Smith JA, Williams SC, Harper WM, Gregg PJ. Lower limb arthroplasty complicated by deep venous thrombosis. Prevalence and subjective outcome. J Bone Joint Surg Br. 2004; 86(1):99–101. http://www.ncbi.nlm.nih.gov/pubmed/14765874. Accessed August 15, 2017.
21. Malviya A, Martin K, Harper I, et al. Enhanced recovery program for hip and knee replacement reduces death rate. Acta Orthop. 2011; 82(5):577–581. doi:10.3109/17453674.2011.618911
22. Cronin C, Green C, Wingate T. Waitematā DHB ERAS Orthopaedic National Collaborative - Fractured Neck of Femur and Elective Hip/Knee Arthroplasty Project - Final Storyboard, January 2015.; 2015.
23. Pebanco GDO, Kaiser SA, Haines ST. New Pharmacologic Methods to Prevent Venous Thromboembolism in Older Adults: A Meta-Analysis. Ann Pharmacother. 2013; 47(5):605–616. doi:10.1345/aph.1R247
24. Stowers MDJ, Manuopangai L, Hill AG, et al. Enhanced Recovery after Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016; 86(6):475–479. doi:10.1111/ans.13538
25. Christelis N, Wallace S, Sage CE, et al. An enhanced recovery after surgery program for hip and knee arthroplasty. Med J Aust. 2015; 202(7):363–369.
26. Drescher FS, Sirovich BE, Lee A, et al. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: A systematic review and meta-analysis. J Hosp Med. 2014; 9(9). doi:10.1002/jhm.2224
27. Wilson DGG, Poole WEC, Chauhan SK, Rogers BA. Systematic review of aspirin for thromboprophylaxis in modern elective total hip and knee arthroplasty. Bone Joint J. 2016; 98-B(8). doi:10.1302/0301-620X.98B8.36957
28. Chu JN, Maselli J, Auerbach AD, Fang MC. The risk of venous thromboembolism with aspirin compared to anticoagulants after hip and knee arthroplasty. Thromb Res. 2017; 155:65–71. doi:10.1016/j.thromres.2017.04.012
29. Bala A, Huddleston JI, Goodman SB, et al. Venous Thromboembolism Prophylaxis after TKA: Aspirin, Warfarin, Enoxaparin, or Factor Xa Inhibitors? Clin Orthop Relat Res. 2017; 475(9):2205–2213. doi:10.1007/s11999-017-5394-6
30. Barrack RL. Current guidelines for total joint VTE prophylaxis: dawn of a new day. J Bone Joint Surg Br. 2012; 94(11 Suppl A):3–7. doi:10.1302/0301-620X.94B11.30824
