ARTICLE
Vol. 133 No. 1511 |
Vitamin D concentrations in New Zealanders with and without inflammatory bowel disease: do they differ?
Vitamin D (25(OH)D or calcidiol) is a fat soluble steroid that plays a major role in bone health through the regulation of serum calcium and phosphate concentrations.
Full article available to subscribers
Vitamin D (25(OH)D or calcidiol) is a fat soluble steroid that plays a major role in bone health through the regulation of serum calcium and phosphate concentrations.1 While the importance of vitamin D to bone health is well established, clear evidence of this hormone’s involvement in immune function has come to light following the discovery of vitamin D receptor expression by a number of immune cell types,2–4 and, similarly, local production of the active vitamin D metabolite calcitriol by certain immune cells.5 Adequate vitamin D concentrations also appear to be important in the prevention of diseases such as cancer, heart disease and immune system mediated diseases.6,7
Two such diseases are Crohn’s disease (CD) and ulcerative colitis (UC), collectively termed inflammatory bowel disease (IBD).8 A link between IBD and vitamin D was proposed in response to the geographical distribution of IBD with the greatest incidences observed in regions furthest North and South of the equator,99 namely New Zealand,10 Canada,11 South Australia12 Iceland13 and Sweden.14 This latitudinal trend is mirrored in other risk factors implicated in IBD including greater industrialisation, extent of development and European ethnicity.9 Moreover, in regions of these latitudes, the combined effect of a greater solar zenith angle, colder temperatures and a conscious reduction in sunlight exposure to reduce skin cancer risk,1,15–17 led to significantly lower vitamin D concentrations than those observed closer to the equator.
New Zealand has one of the highest reported rates of IBD worldwide, and unlike many Western countries where the number of new diagnoses has plateaued, the incidence and prevalence continue to rise.10,18 In the last two decades researchers have identified a number of genes strongly associated with IBD, especially CD.19 However, heritability does not account for all instances and the current consensus is that environmental and immune factors are also involved. New Zealand’s high IBD incidence, elongated shape and southern geographical position make it an ideal location to explore the possible relationship between vitamin D levels and IBD. The aim of this study was to determine the vitamin D concentration of New Zealand patients with IBD and to establish whether they differ from those of controls and are related to disease activity.
Methods
Questionnaire
Participants aged 16 years and above, diagnosed with IBD, or controls with no family history of gastrointestinal disorders, responded to advertisements placed in gastroenterology clinics and at community IBD support organisations located throughout New Zealand. Participants were asked to complete a self-administered retrospective questionnaire developed expressly for this study (see Appendix), concerning demographic data, residence location, holiday history in the previous 12 months, sunlight exposure habits, time spent outdoors per week, vitamin D testing in the previous 12 months and vitamin D supplementation in the previous six months. Residence location was coded by island (North or South), then by latitude (<37.5°S, 37.5–40°S, 40–42.5°S, 42.5–45°S, and >45°S). Sunlight exposure habits comprised sunblock use, protective clothing worn, shade seeking behaviour, sunbathing and sunburn. Patients with IBD were asked additional questions about disease history. Questionnaires were provided in hardcopy or via a secure online survey platform (surveymonkey.com).
Sample collection
Participants residing in or close to 10 major cities and towns throughout the length of New Zealand were invited to take part in the second part of the study by providing a small blood spot sample for serum vitamin D (25(OH)D) measurement. Exclusion criteria included individuals with a blood borne disease or not living predominantly in New Zealand in the previous 24 months. To obtain the sample, a finger lance was used and a minimum of three blood spots were collected from the second or third finger of the non-dominant hand. The blood spot collection cards were air-dried and stored according to the manufacturer’s instructions (ZRT Laboratory, Oregon, US). Samples were collected by the same researcher over two calendar months to reduce potential sampling method disparities and to minimise seasonal differences in vitamin D concentration. Participants consenting to a blood spot sample, and residing in one of two cities, were also invited to take part in a sub-study where a 5ml blood sample was taken by venepuncture to measure serum vitamin D in order to validate the blood spot method.
Blood spot validation
Serum vitamin D levels were measured in dried blood spots using liquid chromatography tandem mass spectrophotometry (LC-MS/MS), and in venous blood samples using high-performance liquid chromatography (HPLC) tandem mass spectrometry (Canterbury Health Laboratories, Christchurch, New Zealand).
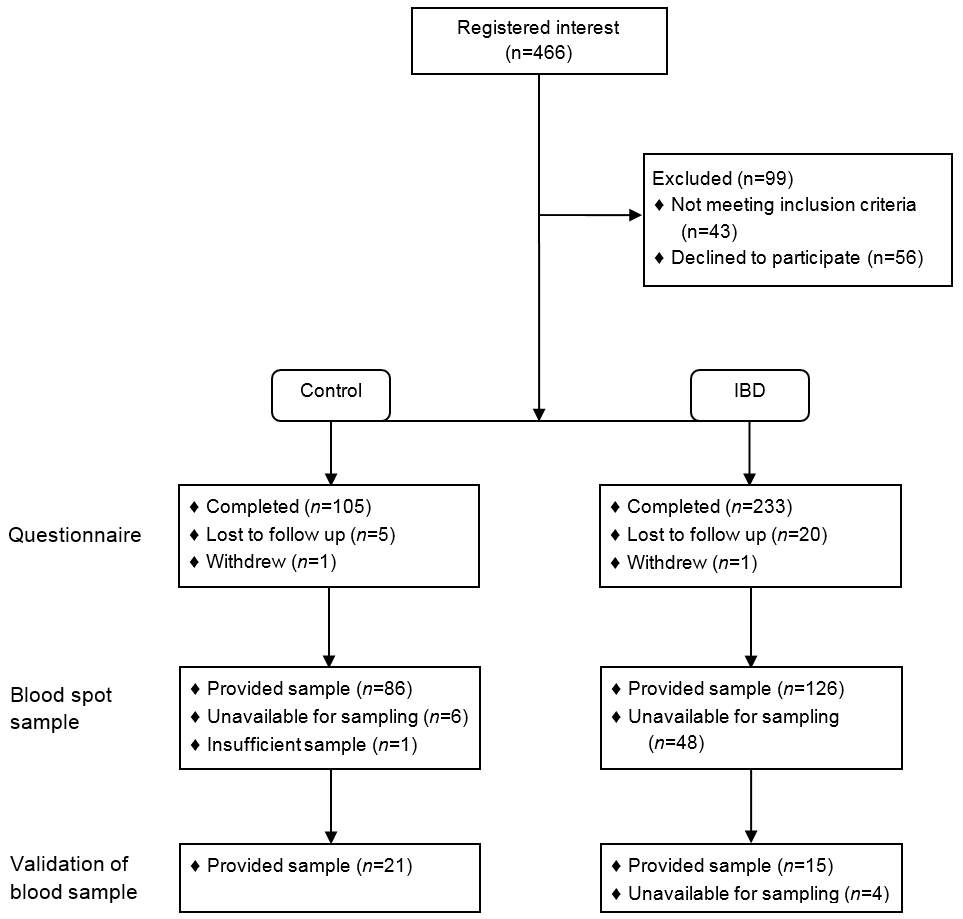
Figure 1: Inclusion and completion of controls and patients with IBD.
Ethics
The protocol for this study was approved by the Massey University Human Ethics Committee: Southern A, Palmerston North, New Zealand (MUHEC Reference 13/58). All study participants provided informed consent.
Statistical analysis
Differences in vitamin D concentration between patients with IBD and controls, and between patients with CD and UC, were analysed using ANOVA TEST (SAS 9.2, North Carolina, US). A p-value of <0.05 was considered statistically significant for all data analysis.
For analysis of vitamin D status, the ranges recommended by the Working Group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia, were applied; severe deficiency <12.5nmol/L, moderate deficiency 12.5–25nmol/L, mild deficiency 25–50nmol/L, and adequacy >50nmol/L.20 Pearson’s Chi-square test was used to test for association between vitamin D status and participant groups; patients with IBD and controls, and between IBD subgroups (CD and UC) (Minitab 17.3.1, Pennsylvania, US).
A linear regression model was applied to vitamin D values from venous blood samples and their corresponding blood spot values (Minitab 17.3.1, Pennsylvania, US). A correction factor was derived from the regression equation (y=10.36+1.1444x) and applied to all blood spot values.
Results
Demographic and participant characteristics
A total of 198 participants completed the questionnaire and provided a blood spot sample, 81 controls, 79 patients with CD, 31 with UC and seven with IBDU.
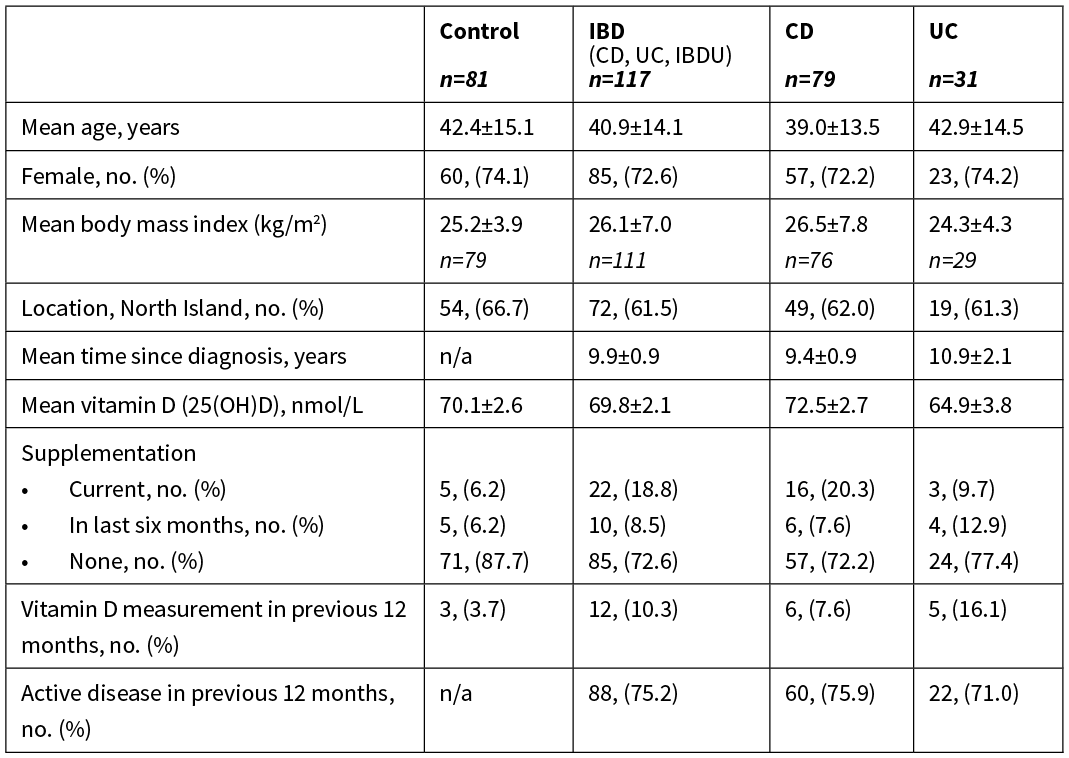
Table 1: Baseline characteristics of the 198 participants.
Vitamin D
No significant difference was observed between the mean vitamin D concentration of patients with IBD and controls, or between IBD subgroups (CD and UC). More than three quarters of patients with IBD and controls had vitamin D concentrations considered adequate (>50nmol/L) (Table 2). Mild vitamin D deficiency (25–50nmol/L) percentages were comparable between patients with IBD and controls, however a greater difference was observed between IBD subgroups. Moderate vitamin D deficiency (12–25nmol/L) was only observed in patients with IBD, and no participants were considered severely vitamin D deficient (<12.5nmol/L). The difference in vitamin D status between patients with IBD and controls, and between IBD subgroups, was not significant.
Table 2: Vitamin D status of controls, patients with IBD, CD and UC.
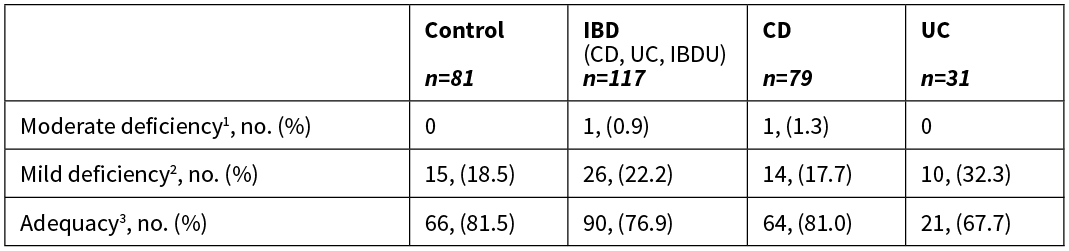
1 12.5-25nmol/L, 2 25–50nmol/L, 3 >50nmol/L.
Vitamin D concentration was significantly higher in patients with IBD, but not controls, who reported increased sunlight exposure on holiday in the previous 12 months (62.0 v 76.3, p=0.001), current supplementation (63.9 v 86.9, p<0.001) or supplementation within the previous six months (63.9 v 81.8, p<0.018). When the effect of increased sunlight exposure on holiday in the previous 12 months was analysed by IBD subgroup, the difference only remained significant in patients with CD (65.0 v 78.4, p=0.011). When the effect of supplementation was analysed by IBD subgroup, the difference associated with current supplementation remained significant in patients with CD (66.8 v 87.9, p<0.001) and in patients with UC (59.7 v 91.5, p=0.018), while the difference associated with previous supplementation only remained significant in patients with CD (66.8 v 85.5, p=0.046). No differences were observed between vitamin D concentration and time spent in sunlight per week, sunlight exposure habits, location or latitude.
Figure 2: Effect of supplementation (current or previous six months) on serum vitamin D concentration by participant group.
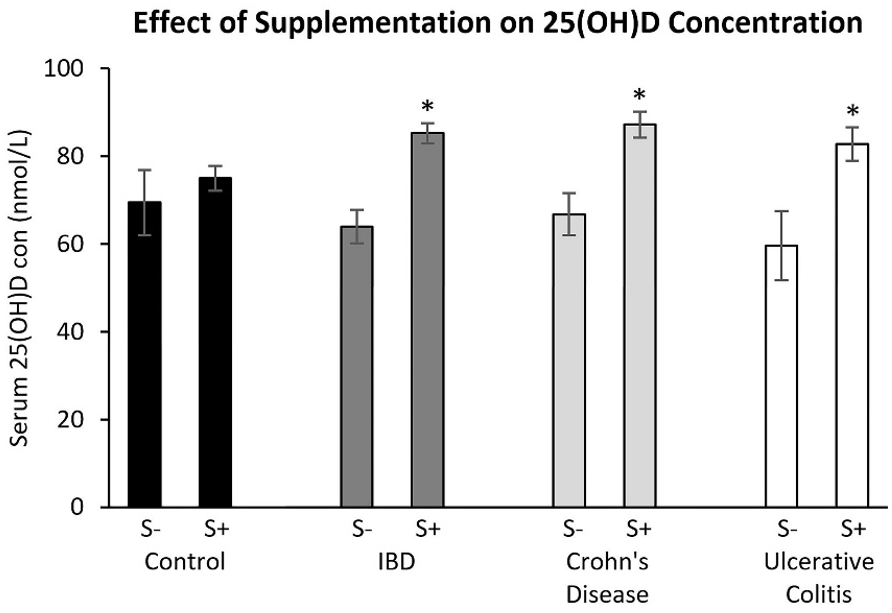
S- no supplementation, S+ supplementationBars marked with * are significantly (p<0.05) different to no supplementation within the same participant group. NB Vitamin D for patients diagnosed with both CD and UC, or IBDU were not analysed individually due to insufficient numbers, they were however included in IBD cohort analyses.
Disease activity
A significant difference in vitamin D concentration was determined between patients with IBD that reported disease activity in the previous 12 months and those that reported remaining in remission only when supplementation was take into account (Table 3).
Table 3: Difference between vitamin D (25(OH)D) concentration and disease activity in the previous 12 months by vitamin D supplementation.
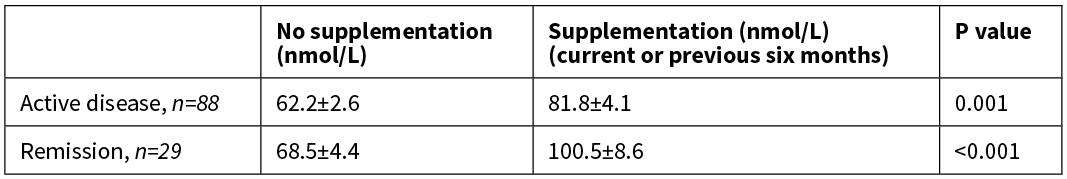
When analysed by IBD subgroup, the mean vitamin D concentration remained significantly lower in patients with CD that had reported disease activity in the previous 12 months (68.6 v 84.6, p=0.008) compared to those that reported remaining in remission, irrespective of supplementation. There was no difference in patients with UC.
Blood spot method validation
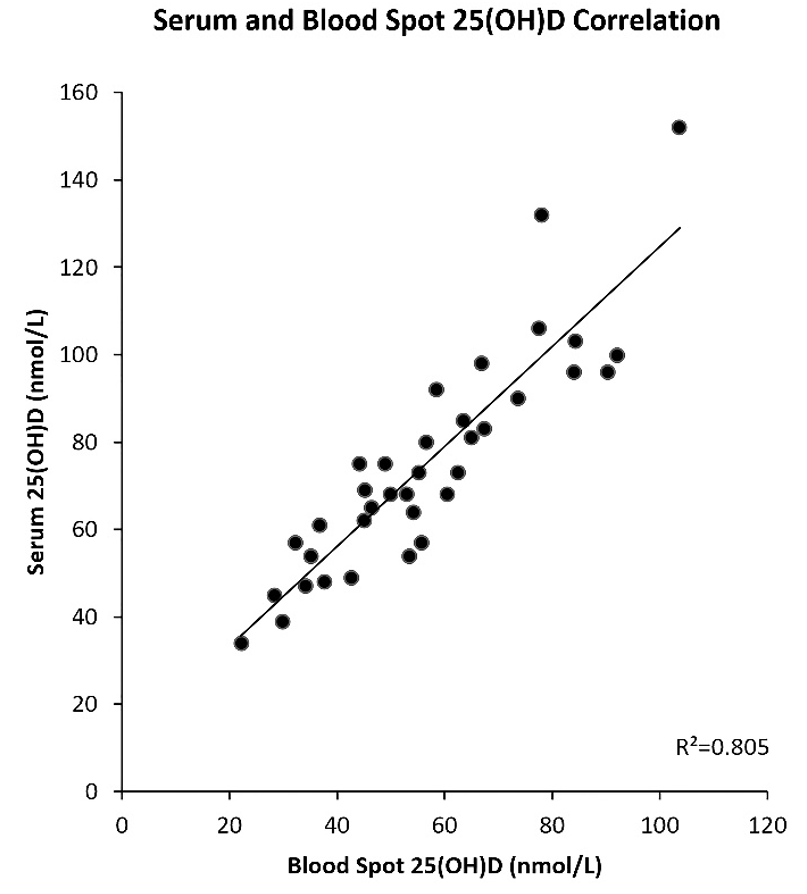
A subset of participants with and without IBD provided a venous blood sample for vitamin D measurement in addition to their blood spot sample (n=36). The mean vitamin D concentration in this subset was 69.9+23.4nmol/L measured in blood spots and 75.0+25.1nmol/L measured in venous blood samples. The blood spot values were lower than their corresponding venous blood sample values and were closely correlated (r2=0.805) (Figure 3).
Figure 3: Correlation between vitamin D measure in blood spot and venous blood samples.
Discussion
The mean vitamin D concentration in this cohort, measured in blood spot samples collected over eight weeks (April–June), was 69.9nmol/L, a level that correlates well with the month standardised national mean of 63.0nmol/L as reported in the New Zealand Adult Nutrition Survey 2008/09.21 This value also falls between the mean concentrations reported in two other New Zealand studies; 53 and 63nmol/L for female and male participants aged 15 years and over, from samples collected nationwide during Autumn (March–May) 1997,22 and 85nmol/L in South Island participants aged 18 years and over in samples collected during late summer (February) 2005.23 Overall, there was no significant difference between the mean vitamin D concentration of controls and patients with IBD, or between IBD subgroups. Accordingly, the percentage of participants considered to have adequate, mildly deficient or moderately deficient vitamin D concentrations was not significantly different between participant groups or subgroups. As discrepancies exist between vitamin D status terminology and the 25(OH)D parameters associated with each status, it is difficult to compare these findings with similar research. However, a meta-analysis of observational studies demonstrated that the odds ratio of being vitamin D deficient (<50nmol/L) compared to controls is 1.63 in CD and 2.28 in UC.24 In line with this data, a greater proportion of mild vitamin D deficiency was observed in patients with UC than those with CD, 32.3% and 17.7% respectively.
The percentage of patients with IBD who reported taking vitamin D supplementation was three-fold greater than controls, and two-fold greater in patients with CD than in patients with UC. Vitamin D concentrations were significantly higher in patients with IBD, but not controls, who reported supplementation currently or in the previous six months. This is unsurprising given the daily dose of 100–1,000IU reported by the controls, compared to 284–7,143IU reported by patients with IBD. While the total number of individuals reporting supplementation was too small to allow further statistical analysis, based on the variance in supplement use and dose, we might have expected a greater difference between the mean vitamin D concentrations. Comparison of the mean vitamin D concentration in patients with IBD, and in controls, reporting no supplementation revealed no significant difference, suggesting a considerable imbalance between oral intakes and ensuing serum concentration likely attributable to impaired nutrient absorption associated with inflammation, notably fat malabsorption,25,26 or having undergone small intestinal resection.27 Researchers have also demonstrated impaired absorption of supplemental vitamin D in clinically quiescent patients with CD compared to controls, a 30% reduction across all patients, and 20% in those without a resection, thus another mechanism may be involved.28 Genetic factors could influence vitamin D concentrations as polymorphisms of the genes that encode the vitamin D binding protein (VDBP) are reported to influence vitamin D concentrations,29 and expression of VDBP genetic variants has been demonstrated to differ between patients with IBD and controls.30
Once adjusted for supplementation, a significant difference in vitamin D concentration was observed between patients with IBD who reported disease activity in the previous 12 months and those that reported remaining in remission. When analysed by IBD subgroup, the difference remained significant only in patients with CD irrespective of supplementation. This may reflect a stronger association between vitamin D concentration and CD, a relationship proposed in the Nurses’ Health Study.31 Alternatively, the present study may not have been adequately powered to investigate a difference in vitamin D concentration between patients with UC reporting active disease and patients with UC reporting remission. Differences in supplementation could potentially explain this observation, as of the patients with UC in remission, 0% reported current supplementation and 25% reported supplementation in the previous six months, compared to 19% and 33% respectively of the equivalent patients with CD.
While the leading source of vitamin D is skin exposure to UV B radiation,5 the only UV exposure factor that had an effect on vitamin D concentration was seen in patients with IBD who reported increased sunlight exposure on holiday in the previous 12 months. Unlike other studies, no effect of latitude on vitamin D concentrations was observed,21,22,32 though a review of global vitamin D status demonstrates this relationship is less marked now than in earlier studies.15 A discernible effect of latitude may also have been obscured by supplement use and dose. No effect was observed between vitamin D concentration and sunlight exposure score, and patients with IBD and controls produced similar scores. This is surprising, as some routinely prescribed IBD medications are associated with increased skin cancer risk, namely azathioprine and mercaptopurine, thus patients prescribed these medications are advised to limit UV exposure.33 Closer inspection revealed that while the patients with IBD reported greater sunscreen reapplication compliance and shade seeking, this was counteracted by lower sunhat use and a higher incidence of sunburn.
Finally, in the present study a close correlation was observed between the two measures of serum vitamin D; blood spot assay using LC-MS/MS and venous blood samples using HPLC. In agreement with other work, the vitamin D concentrations measured in blood spot LC-MS/MS assay were lower than their corresponding HPLC assay values.34,35 This difference may in part be explained by variation between the assay methods, a recognised obstacle that led to the development of the Vitamin D External Quality Assessment Scheme (DEQAS), a scheme formed in 1989 to appraise vitamin D assay reliability.36,37 It has also been suggested that assay variability may be attributable to either one or a combination of three factors; blood spot volume less than 50µl, differences in blood volume absorption due to variations in filter paper weight and the location of the punched spot on the paper.38 While obtaining adequate blood spot volume can be improved by collecting additional spots, the latter two factors may be more difficult to control.
Conclusion
Vitamin D concentrations were not different between New Zealand patients with IBD compared to controls irrespective of marked differences in supplement use and dose. This likely presents a challenge for reaching and maintaining adequate vitamin D concentrations in patients with IBD who have limited UV exposure or are not receiving regular supplementation. In patients with CD, a history of active disease in the previous 12 months was significantly associated with lower vitamin D concentration compared to patients who reported remaining in remission. Although this difference was not observed in patients with UC, vitamin D supplementation is safe, effective and inexpensive39 and may have implications for reducing disease activity recurrence. Vitamin D supplementation should be recommended to patients with IBD by their healthcare providers, especially to patients with a recent history of active disease and those being treated with sun-sensitising medications.
Study limitations
The present study had several limitations. Samples were collected from April through June when vitamin D concentrations are expected to be moderately high following summer and thus do not indicate nadir concentrations. Obtaining this information would have been useful for demonstrating the extent of both vitamin D inadequacy and deficiency in this at-risk population group. A retrospective questionnaire was used to collect data about sunlight exposure, vitamin D supplementation and disease activity. The sunlight exposure habits assessed were assumed to be of equal contribution to UV exposure due to the lack of a suitable validated questionnaire. The collection of disease activity history over a period of 12 months prevented the use of a validated disease activity index; however, using an index would have been time consuming and may have reduced the number of responses. Lastly, the sample size, particularly the number of patients with UC, may have limited the findings.
Aim
Patients with inflammatory bowel disease (IBD), Crohn’s disease (CD) or ulcerative colitis (UC) are at risk of low vitamin D owing to reduced absorption, medication-associated sunlight exposure restrictions and/or increased requirements due to inflammation. This study aimed to determine if the serum vitamin D concentration of New Zealand IBD patients relates to disease activity and differs from controls.
Methods
Data concerning demographics, sunlight exposure, vitamin D supplementation and disease activity were collected using a retrospective questionnaire. Serum vitamin D concentrations were measured in dried blood spots and validated against blood samples in a participant sub-group.
Results
Vitamin D concentration was significantly increased by supplementation (82.8 v 66.4nmol/L, p<0.001) and sunlight exposure while on holiday (75.2 v 63.7nmol/L, p<0.001). Patients with CD who reported active disease in the last year had significantly lower vitamin D concentrations (68.6 v 84.6nmol/L, p=0.008) than those who reported remaining in remission.
Conclusion
In this cohort of New Zealand residents, mean vitamin D of patients with IBD was not different from controls. In patients with CD, recent disease activity was significantly associated with lower vitamin D. The use of vitamin D supplementation may have implications for reducing disease activity occurrence in patients with CD.
Authors
Hannah Morton, PhD student, School of Food and Advanced Technology, College of Sciences, Massey University, Palmerston North; Kevin C Pedley, Hon Associate Professor, Massey University, Palmerston North; Robin JC Stewart, Lecturer, School of Applied Science, Faculty of Health and Sciences, Universal College of Learning, Palmerston North; Jane Coad, Professor, School of Food and Advanced Technology, College of Sciences, Massey University, Palmerston North.Correspondence
Professor Jane Coad, School of Food and Advanced Technology, College of Sciences, Massey University, Palmerston North 4442.Correspondence email
j.coad@massey.ac.nzCompeting interests
Dr Coad reports grants from Massey University School of Food and Advanced Technology, grants from Graduate Women New Zealand, awarded to Hannah Morton (PhD student) during the conduct of the study. Ms Morton reports grants from Massey University School of Food and Advanced Technology, grants from Graduate Women New Zealand, during the conduct of the study.1. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011; 91:115–24.
2. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983; 221:1181–3.
3. Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009; 70:345–52.
4. Takahashi K, Nakayama Y, Horiuchi H, et al. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol Immunotoxicol. 2002; 24:335–47.
5. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012; 71:50–61.
6. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004; 79:362-71.
7. Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004; 80:1717–20S.
8. Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005; 2:308–15.
9. Economou M, Pappas G. New global map of Crohn’s disease: Genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis. 2008; 14:709–20.
10. Su HY, Gupta V, Day AS, Gearry RB. Rising Incidence of Inflammatory Bowel Disease in Canterbury, New Zealand. Inflamm Bowel Dis. 2016; 22:2238–44.
11. Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006; 101:1559–68.
12. Wilson J, Hair C, Knight R, et al. High incidence of inflammatory bowel disease in Australia: a prospective population-based Australian incidence study. Inflamm Bowel Dis. 2010; 16:1550–6.
13. Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996; 39:690–7.
14. Rönnblom A, Samuelsson SM, Ekbom A. Ulcerative colitis in the county of Uppsala 1945-2007: incidence and clinical characteristics. J Crohns Colitis. 2010; 4:532–6.
15. Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009; 20:1807–20.
16. Kimlin MG. Geographic location and vitamin D synthesis. Mol Aspects Med. 2008; 29:453–61.
17. Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007; 460:213–7.
18. Gismera CS, Aladrén BS. Inflammatory bowel diseases: a disease (s) of modern times? Is incidence still increasing? World J Gastroenterol. 2008; 14:5491–8.
19. de Lange KM, Moutsianas L, Lee JC, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017; 49:256–61.
20. Working Group of the Australian and New Zealand Bone and Mineral Society; Endocrine Society of Australia; Osteoporosis Australia. Vitamin D and adult bone health in Australia and New Zealand: A position statement. Med J Aust. 2005; 182:281–5.
21. Ministry of Health. Vitamin D Status of New Zealand Adults: Findings from the 2008/09 New Zealand Adult Nutrition Survey. 2012. Available from: http://www.health.govt.nz/publication/vitamin-d-status-new-zealand-adults
22. Rockell JE, Skeaff CM, Williams SM, Green TJ. Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos Int. 2006; 17:1382–9.
23. Rockell JE, Skeaff CM, Williams SM, Green TJ. Association between quantitative measures of skin color and plasma 25-hydroxyvitamin D. Osteoporos Int. 2008; 19:1639–42.
24. Del Pinto R, Pietropaoli D, Chandar AK, et al. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015; 21:2708–17.
25. Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985; 42:644–9.
26. Margulies SL, Kurian D, Elliott MS, Han Z. Vitamin D deficiency in patients with intestinal malabsorption syndromes - think in and outside of the gut. J Dig Dis. 2015; 16:617–33.
27. Tajika M, Matsuura A, Nakamura T, et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J Gastroenterol. 2004; 39:527–33.
28. Farraye FA, Nimitphong H, Stucchi A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011; 17:2116–21.
29. Gozdzik A, Zhu J, Wong BY, et al. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011; 127:405–12.
30. Eloranta JJ, Wenger C, Mwinyi J, et al. Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenet Genomics. 2011; 21:559–64.
31. Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012; 142:482–9.
32. van der Mei IA, Ponsonby AL, Engelsen O, et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007; 115:1132–9.
33. Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2014; 109:163–9.
34. Eyles DW, Morley R, Anderson C, et al. The utility of neonatal dried blood spots for the assessment of neonatal vitamin D status. Paediatr Perinat Epidemiol. 2010; 24:303–8.
35. Larkin EK, Gebretsadik T, Koestner N, et al. Agreement of blood spot card measurements of vitamin D levels with serum, whole blood specimen types and a dietary recall instrument. PLoS One. 2011; 6:e16602.
36. Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004; 50:2195–7.
37. Binkley N, Krueger D, Gemar D, Drezner MK. Correlation among 25-hydroxy-vitamin D assays. J Clin Endocrinol Metab. 2008; 93:1804–8.
38. Kvaskoff D, Ko P, Simila HA, Eyles DW. Distribution of 25-hydroxyvitamin D3 in dried blood spots and implications for its quantitation by tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012; 901:47–52.
39. Hlavaty T, Krajcovicova A, Payer J. Vitamin D therapy in inflammatory bowel diseases: who, in what form, and how much? J Crohns Colitis. 2015; 9:198–209.
