ARTICLE
Vol. 133 No. 1509 |
Epidemiology of traumatic spinal cord injury in New Zealand (2007–2016)
A traumatic spinal cord injury (TSCI) is a life-changing event for an individual and their family/whānau. Vast costs are also associated with the extensive treatment, rehabilitation and lost productivity incurred.
Full article available to subscribers
A traumatic spinal cord injury (TSCI) is a life-changing event for an individual and their family/whānau. Vast costs are also associated with the extensive treatment, rehabilitation and lost productivity incurred.1 The incidence for TSCI varies worldwide.2,3 Recent multinational studies also suggest demographics are changing over time, with an increasing median age of TSCI and higher incidence in older adults.3,4
Previously published data on the epidemiology of TSCI in New Zealand is limited but suggests it may have one of the highest rates of TSCI in the western world, particularly among Māori and Pacific Islanders.5,6 Understanding the demographics of this specific cohort will support healthcare planning, shape clinical research priorities and assist patient care to achieve optimal quality of life, social and economic outcomes.
The aim of this study is to utilise the newly established New Zealand Spinal Cord Injury Registry (NZSCIR) to better understand the epidemiology and demographic trends of patients admitted for spinal rehabilitation following TSCI in New Zealand over a 10-year period, from January 2007 to December 2016.
Methods
Patient population and the NZSCIR
Currently two centres provide spinal injury rehabilitation care in New Zealand, the Auckland Spinal Rehabilitation Unit (ASRU) and the Burwood Spinal Unit (BSU, Christchurch). The units were established with the aim that each location will serve half of the total New Zealand population (currently 4.69 million, 2018 census); however, due to the skewed population distribution in New Zealand the geographical catchment areas of each centre varies. The ASRU covers injuries sustained in the upper North Island, while the Burwood Spinal Unit (BSU) covers the lower North Island and the whole of the South Island.
In 2016, the NZSCIR was established in partnership with the Rick Hansen Institute (Canada), to collect relevant data on all patients sustaining a traumatic or non-traumatic SCI (NTSCI) in New Zealand. The NZSCIR prospectively records a minimal data set (MDS) (Table 1), comparable to the Rick Hansen Spinal Cord Injury Registry, for all patients admitted to both New Zealand spinal units and full prospective data on patients who give consent.8 The NZSCIR defines SCI as impairment of the spinal cord or cauda equina function resulting in either a motor or sensory deficit or both.9 To support the aims of this study, retrospective admission data from both rehabilitation units was used to identify all patients admitted from 1 January 2007 to 31 July 2016. MDS data (Table 1) were collected on these patients from electronic and hard copy medical notes and entered into the NZSCIR by three research assistants under the guidance of an NZSCIR Coordinator. Where data was unavailable, the respective fields were left ‘unknown’.
Table 1: NZSCIR minimal data set.
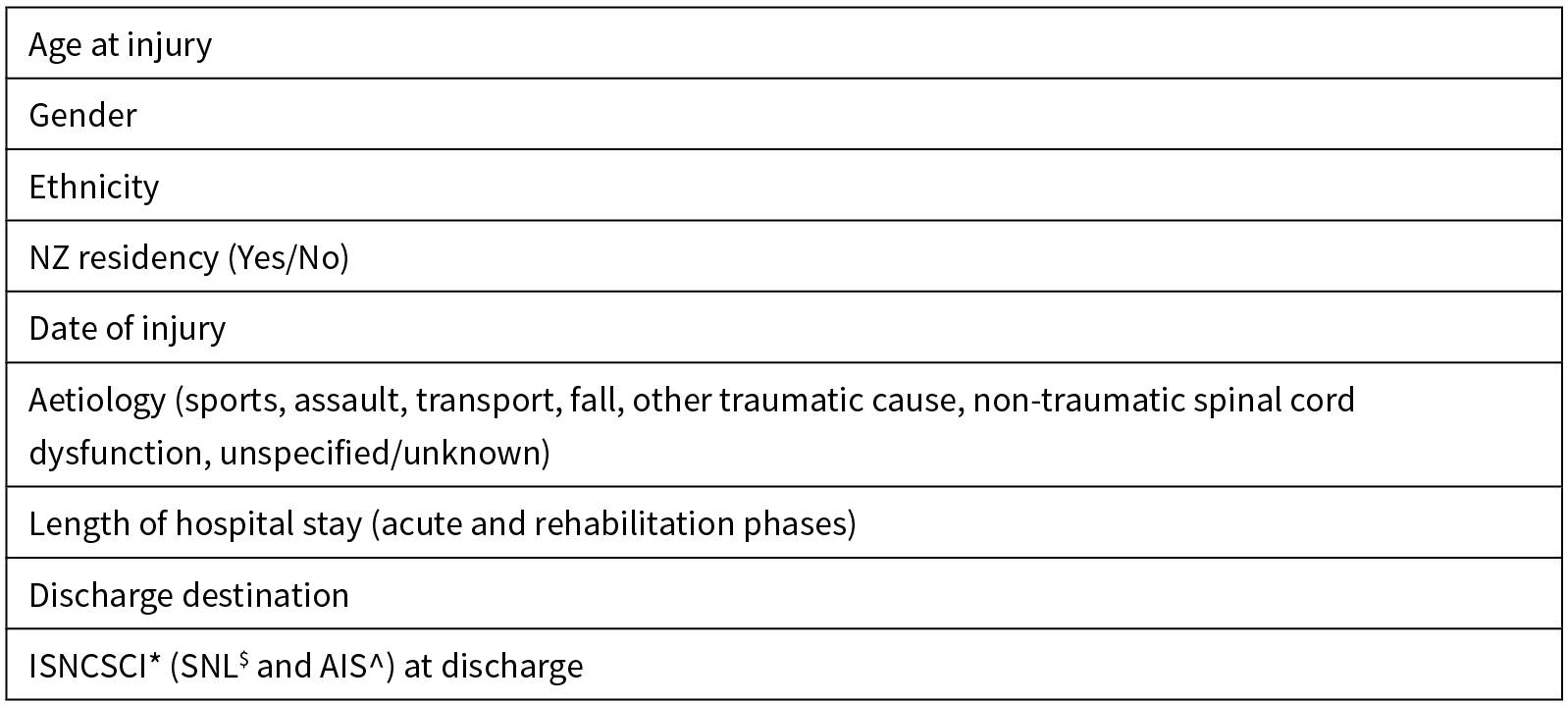
*International Standards for Neurological Classification of Spinal Cord Injury; $Single Neurological Level; ^American Spinal Injury Association (ASIA) Impairment Scale.
These data were combined with five months of prospective MDS data from the NZSCIR, providing a combined 10-year ambispective cohort from January 2007 to December 2016. The inclusion criteria involved adult patients (age >16 years) admitted to either spinal unit for rehabilitation with a new TSCI. Patients with NTSCI were excluded from this study.
Aetiology was coded using the adapted International Classification of External Causes of Injuries (ICECI), as per the International SCI Core Data Set.10 Sports, transport and falls were further classified, including a free text description. ‘Other Traumatic Cause’ includes SCI following medical procedures which directly injured or caused vascular compromise (eg, ischaemia or infarction) to the spinal cord or cauda equina.
Length of hospital stay was recorded in days, from acute admission at any New Zealand hospital to discharge from the spinal rehabilitation unit. International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) on rehabilitation discharge was collected, giving a single neurological level (SNL) and an American Spinal Injury Association Impairment Scale (AIS) grade. Neurological level was categorised anatomically as C1-4, C5-8, Thoracic, Lumbosacral. The AIS is a standardised grading system used to classify the severity (completeness) of neurological injury in individuals with SCI.11 It is based on the testing of power in key muscle (motor) groups (graded as 0 (no movement) to 5 (full strength)), a dermatomal based sensory function examination combined with an anorectal assessment. The 5-point ordinal AIS classifies individuals from “A” (complete SCI) to “E” (normal sensory and motor function) based on this assessment of motor, sensory and anorectal function below the injured neurological level.11 Patients with AIS Grade B injuries have some sensory function preserved but no motor function below the injured neurological level, AIS C injuries have motor function preserved with half the muscles below the injured level having a muscle grade less than 3 (full active movement against gravity but without resistance), while AIS D injuries have a motor grade of 3 or more in at least half of key muscles below the neurological level.11
Analysis
The data were imported from NZSCIR into SPSS V25.0 to produce descriptive demographic and aetiologic summaries for the incident TSCI cases. The incidence rates were calculated using the New Zealand Census data from 2006 and 2013, and 95% confidence intervals calculated using a Poisson approximation.
Ethics approval
The study was approved by the University of Otago Ethics Committee (Health) HD19/029.
Results
Data from 929 patients were collected over the 10-year interval from January 2007 to December 2016.
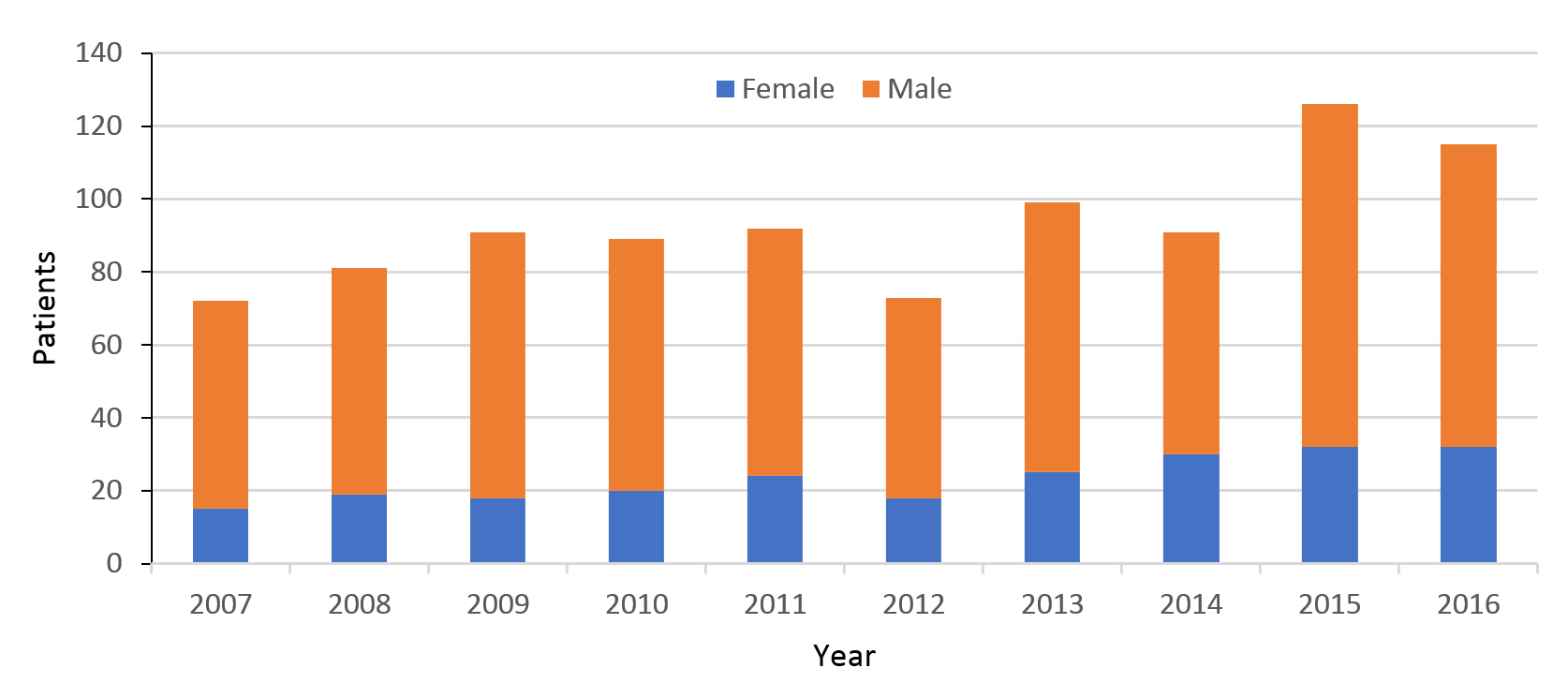
The absolute number of cases of admissions per year ranged from 72 to 126, with a mean of 93. Data showed a trend of increasing numbers per year (Figure 1). The proportion of male to female injuries decreased over the study period, with males accounting for 79% of admissions in 2007 and 72% in 2016. Comparisons of the first and last years of the 10-year period show female admissions had more than doubled (15 in 2007 to 32 in 2016), while males only increased 1.4 times (57 in 2006 to 83 in 2016).
Figure 1: Traumatic spinal cord injury (TSCI) admissions 2007–2016.
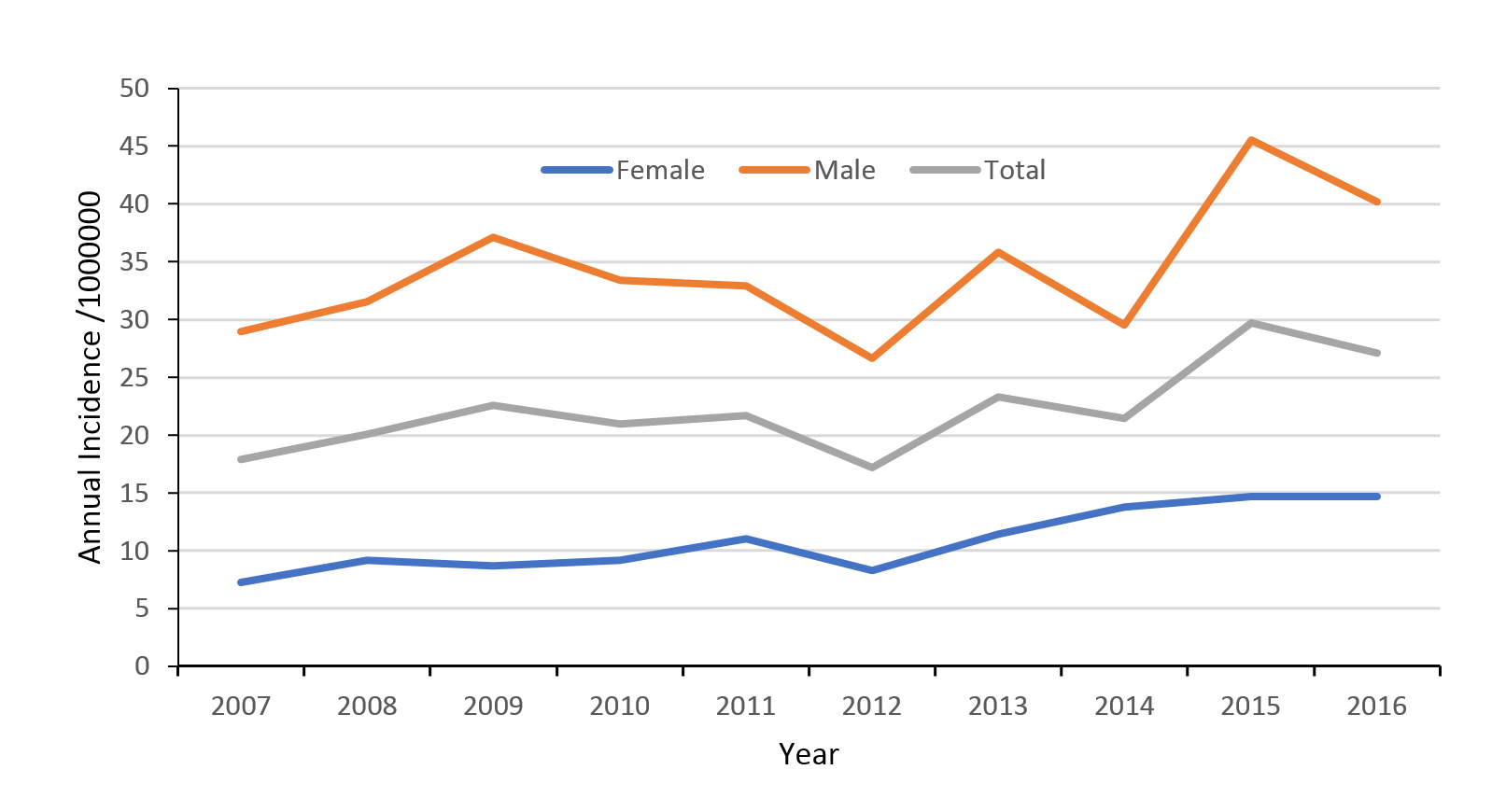
The mean national annual incidence of TSCI was 22 per million people (95% CI 21–24). The mean incidence of TSCI in men was 34 per million (95% CI 32–37) and 11 per million people (95% CI 10–12) in women. Over the 10-year study period, the incidence of TSCI increased on average 6% per year (Figure 2).
Figure 2: Traumatic spinal cord injury (TSCI) incidence rates 2007–2016.
Age
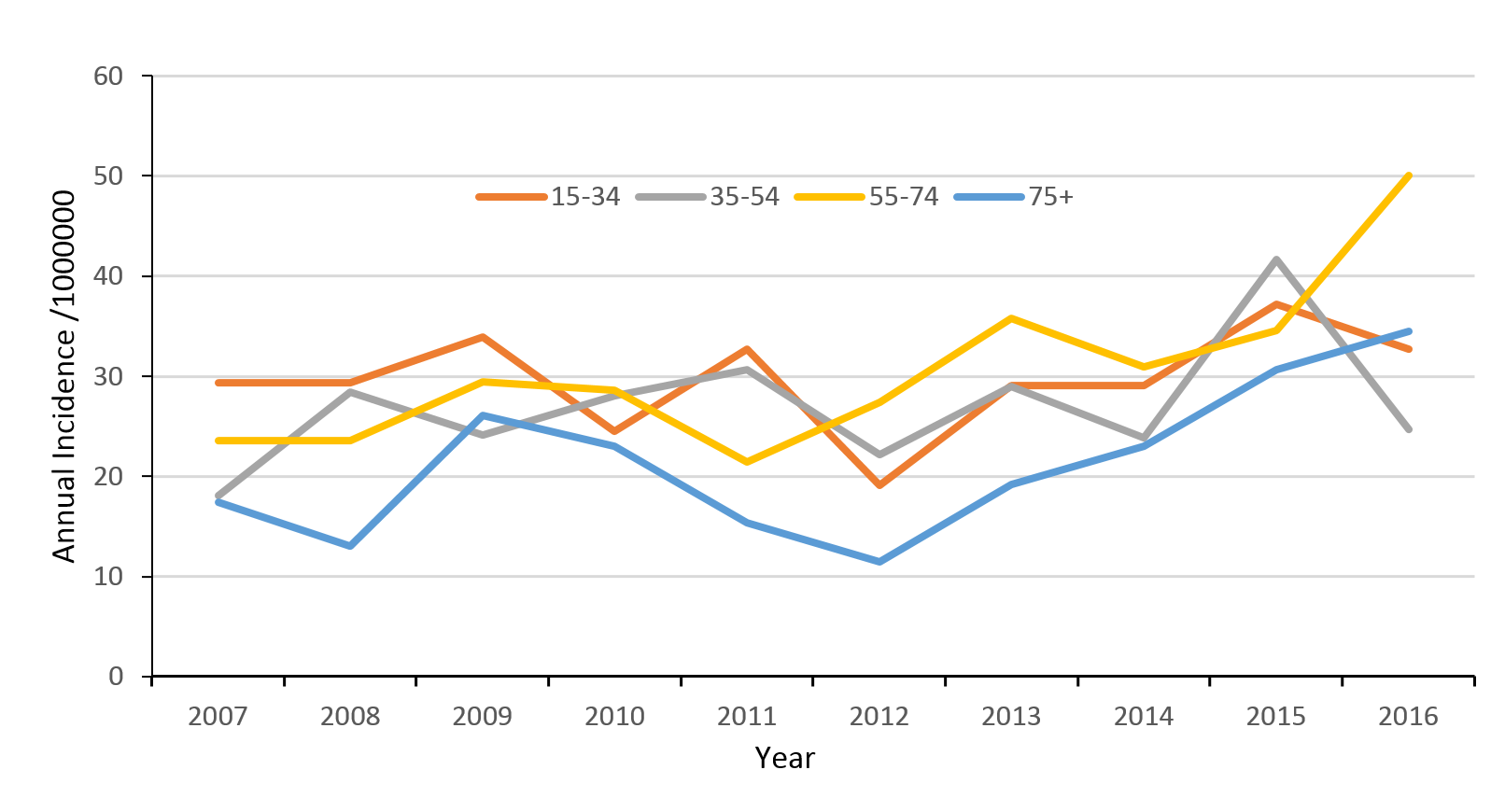
The mean age of patients increased from 43 years to 48 years over the 10-year period. In every age group, there was an increased annual incidence of TSCI, with the greatest increases in people over the age of 55. In the study’s final year (2016) the age bracket of 55–74yr had the highest incidence of all age ranges (Figure 3).
Figure 3: Age-specific traumatic spinal cord injury (TSCI) incidence rates 2007–2016.
Ethnicity
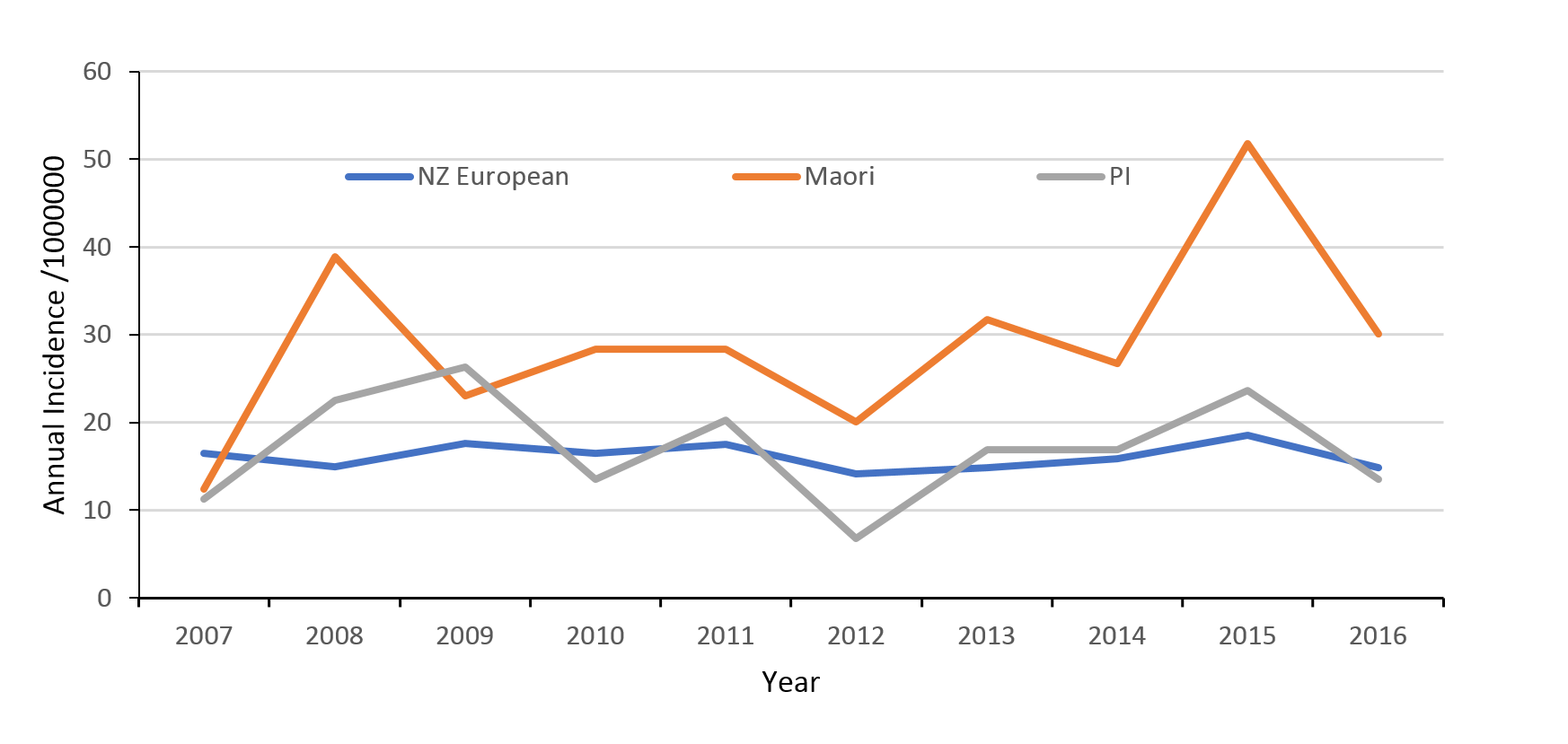
The highest incidence of TSCI was in the Māori population with mean rates of 29 per million people (95% CI 25–34), compared to New Zealand Europeans 16 per million people (95% CI 15–18) and Pacific Islanders 17 per million people (95% CI 13–23) (Figure 4). TSCI in Māori was 1.8 times more frequent than in New Zealand Europeans. The rate of increase in incidence of TSCI in the Māori population was approximately 14% throughout the study period while the incidence among the New Zealand European and Pacific Islander groups remained largely static.
Figure 4: Traumatic spinal cord injury (TSCI) rates by ethnicity.
Aetiology
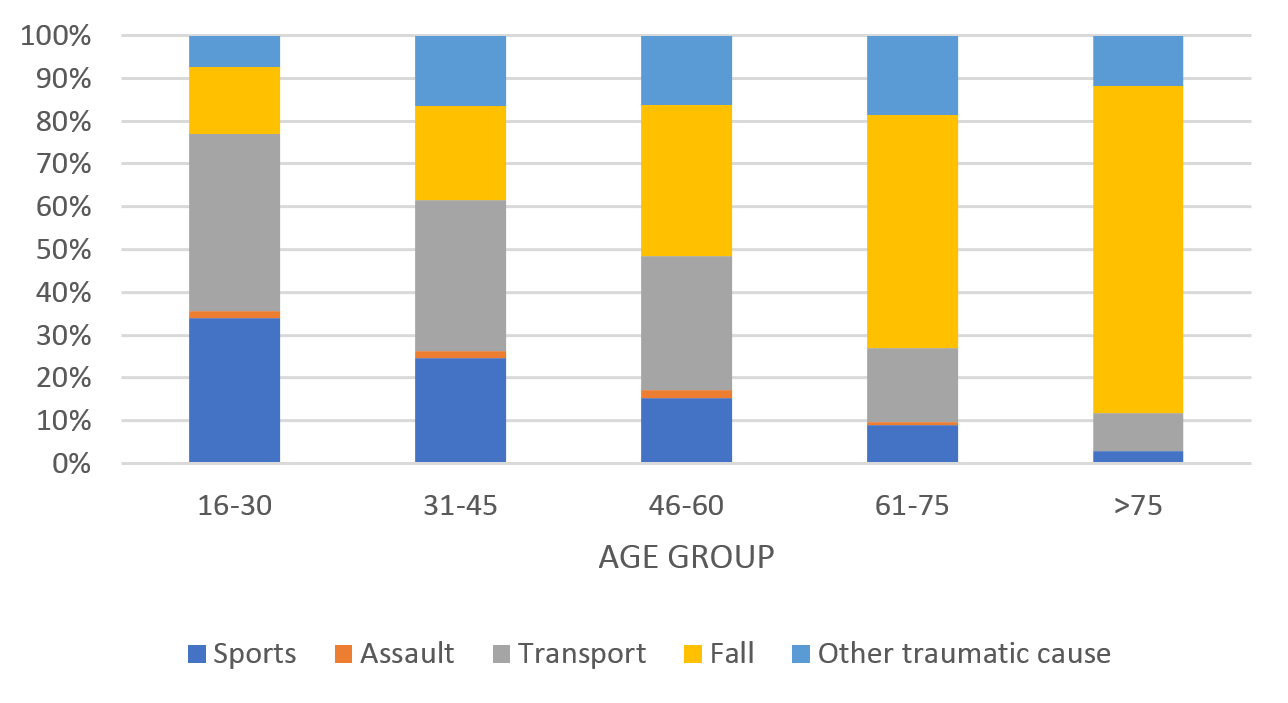
Over the 10-year study period the most common aetiology of TSCI was transport (32%), followed by falls (31%) and sports (21%). Aetiology varied significantly according to age. Motor vehicle crash and sports were the most common mechanisms of injury in younger cohorts and falls was the most prevalent cause in patients over 60 years (Figure 5).
Figure 5: Aetiology of traumatic spinal cord injury (TSCI) by age group.
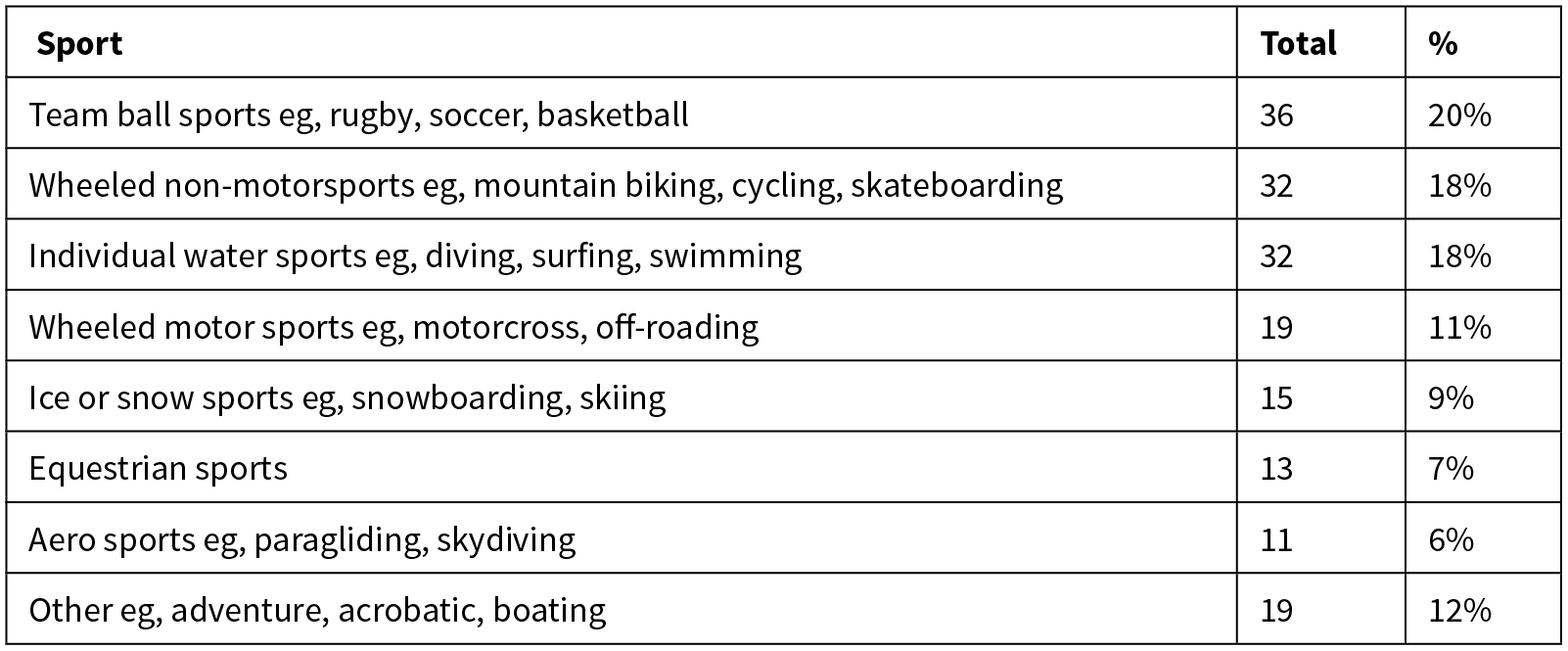
A breakdown of the specific aetiology of sports-related TSCI are shown in Table 2. Team ball sports (eg, rugby) remained the most common sporting cause overall (20%), however rates varied significantly with age. Team ball sports (eg, rugby) was the most common sporting mechanism in the 16–30 age group, causing 31% of sporting injuries, while water sports (eg, diving) was the most common cause in 31–45 year-olds (23%) and wheeled non-motorsports (eg, mountain biking) was most common in ages 46–75 (37%).
Table 2: Detailed sports aetiology.
Surgical intervention
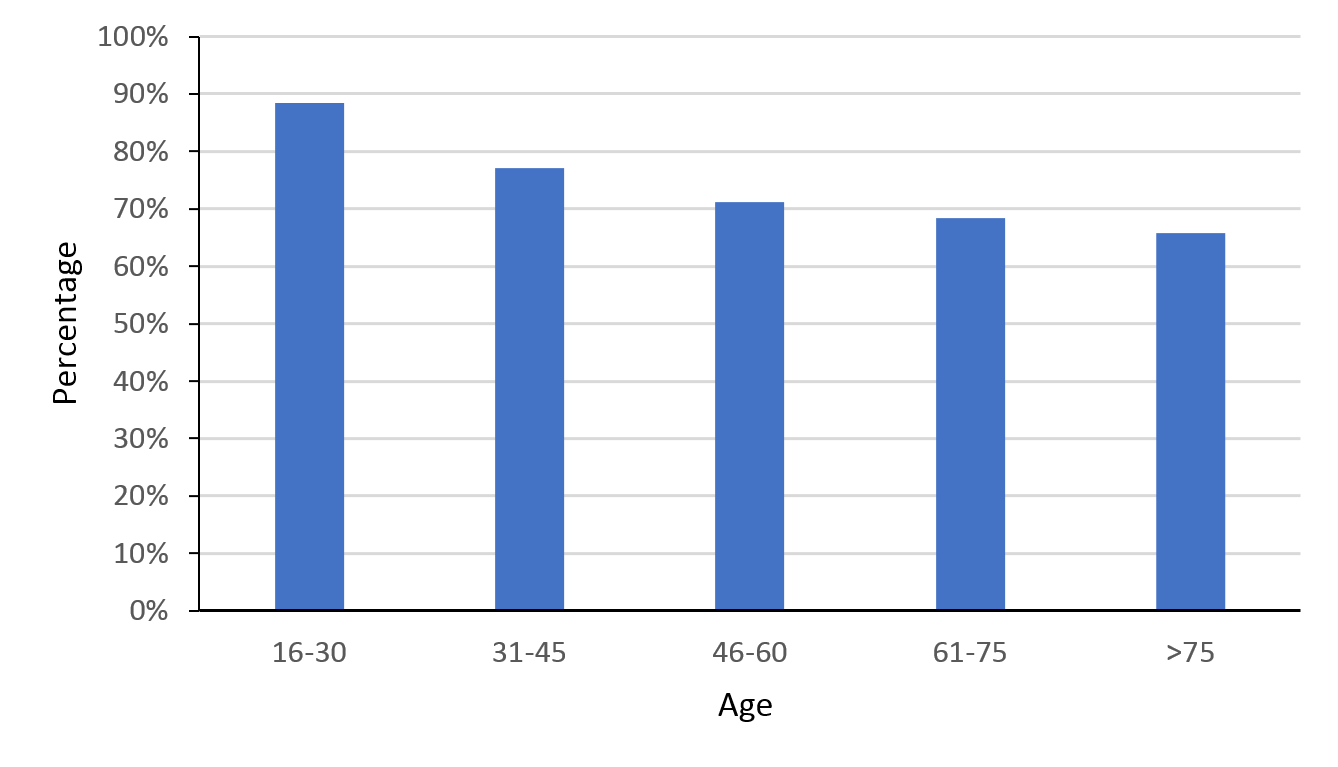
Over the last 10 years the surgical rate remained stable, with 77% (range 66–83%) of patients receiving surgical management. Surgical management was most common in the 16–30 age group (88%). Rates of surgical intervention declined with age (Figure 6).
Figure 6: Percentage of traumatic spinal cord injury (TSCI) patients who underwent surgery.
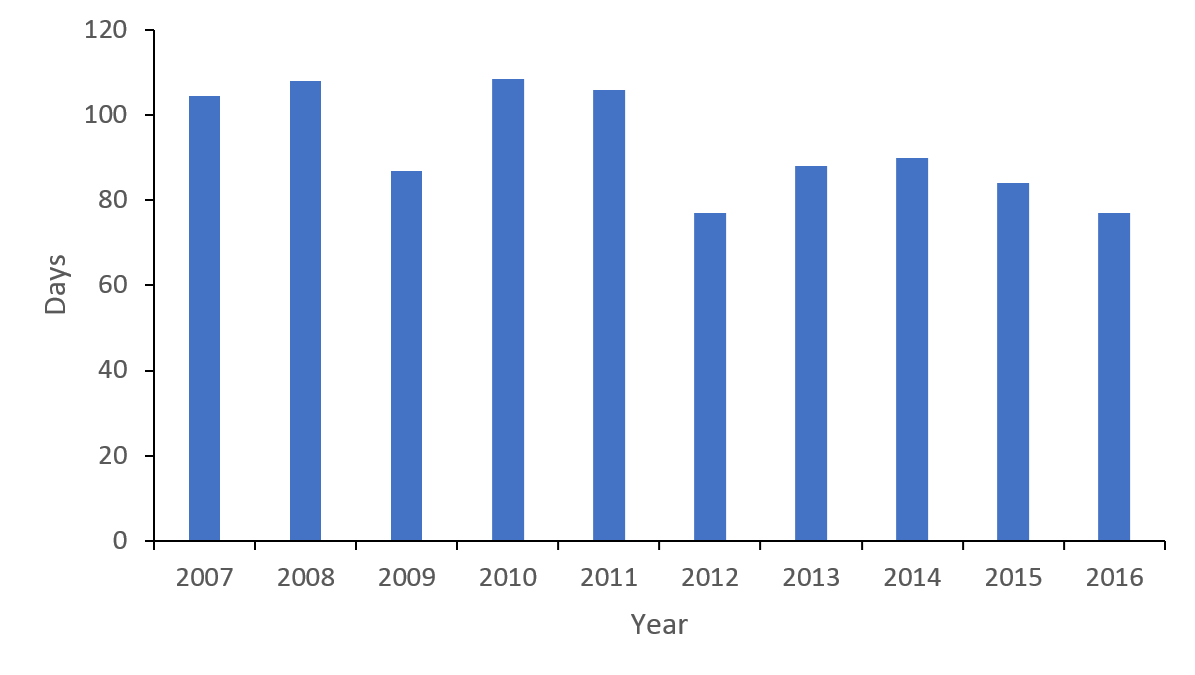
Duration of hospital admission
The length of stay (LOS) reduced from a median of 105 days in 2007 to 77 days in 2016 (Figure 7), a trend evident at both individual rehabilitation centres. The LOS did not appear to be influenced by ethnicity, with New Zealand Europeans (93 days), Māori (96 days) and Pacific Islanders (91 days) sharing similar timeframes.
Figure 7: Median length of stay (days) Traumatic Spinal Cord Injury (TSCI).
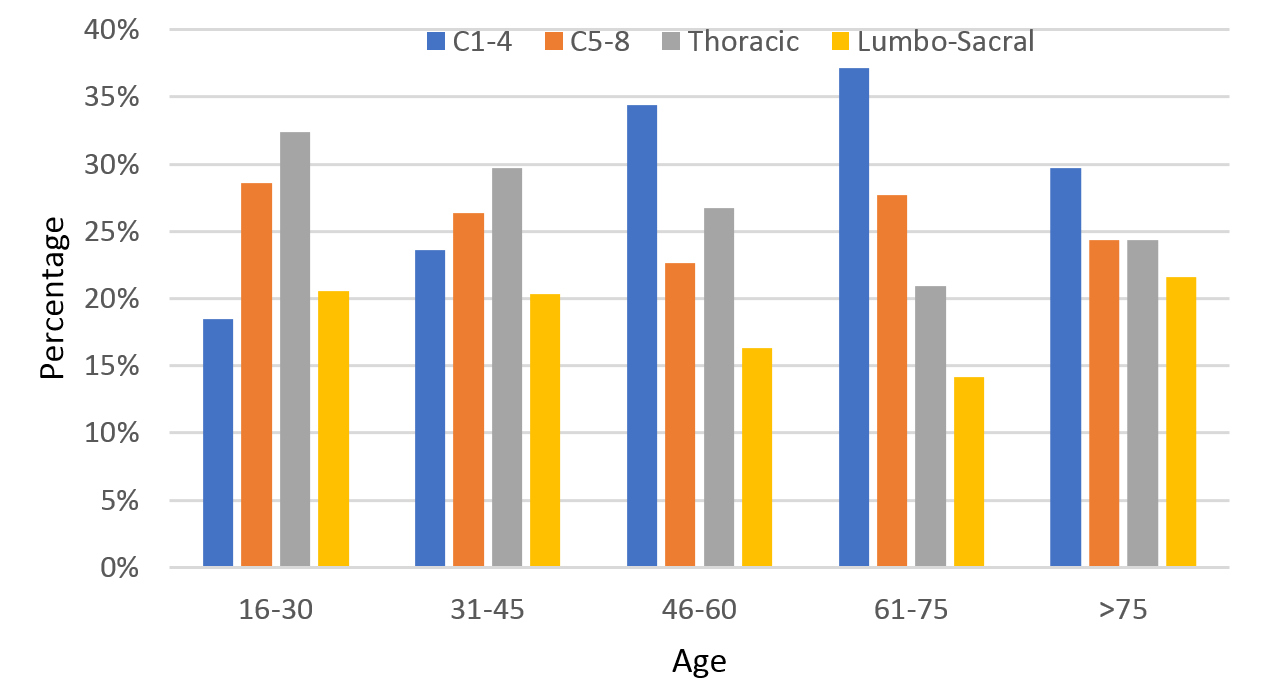
Neurological injury level
Cervical level injuries were most common, accounting for 54% of all injuries (52% C1-4 and 48% C5-8), while thoracic injuries accounted for 28% of TSCI and 18% of patients had a lumbo-sacral injury. Cervical injuries were more common in the older age groups, peaking at 65% (57% C1-4, 43% C5-8) in the 61–75 age group (Figure 8).
Figure 8: Age stratified neurological level of traumatic spinal cord injury (TSCI).
People identifying as Pacific Island and Māori had a higher proportion of cervical injuries, with 76% (54% C1-4, 46% C5-8) of Pacific and 61% (58% C1-4, 41% C5-8) of Māori patients with a cervical injury, compared to only 50% (50% C1-4, 50% C5-8) of New Zealand European patients.
Impairment level on discharge
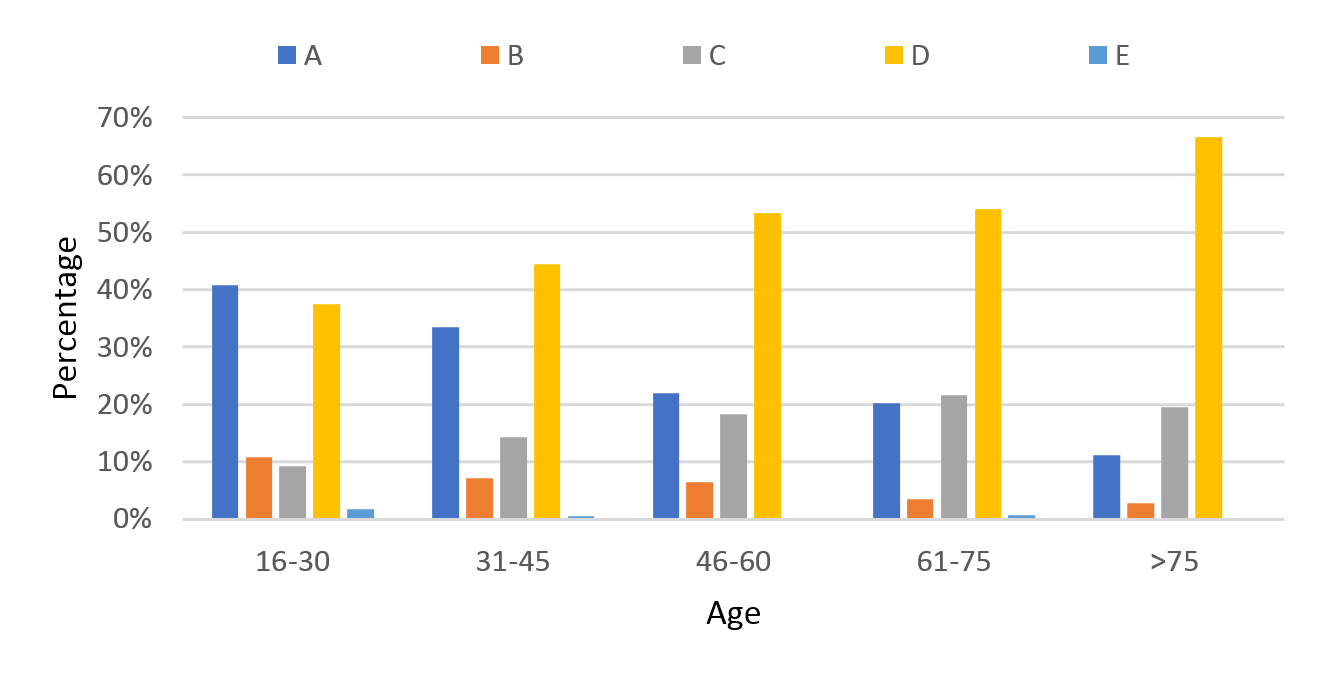
Over the 10-year period there was a decrease in the proportion of patients with an AIS-A impairment score on discharge. In 2007, 40% of patients were discharged with an AIS A, in 2016 this number decreased to 30%. Over the same time period the proportion of patients with AIS D increased from 34 to 49% (Figure 9). AIS A injuries were more common in younger patients (41% of patients aged between 16–30 (Figure 10) compared with the >75 age group (11%).
Figure 9: Percentage of patients by discharge AIS score.
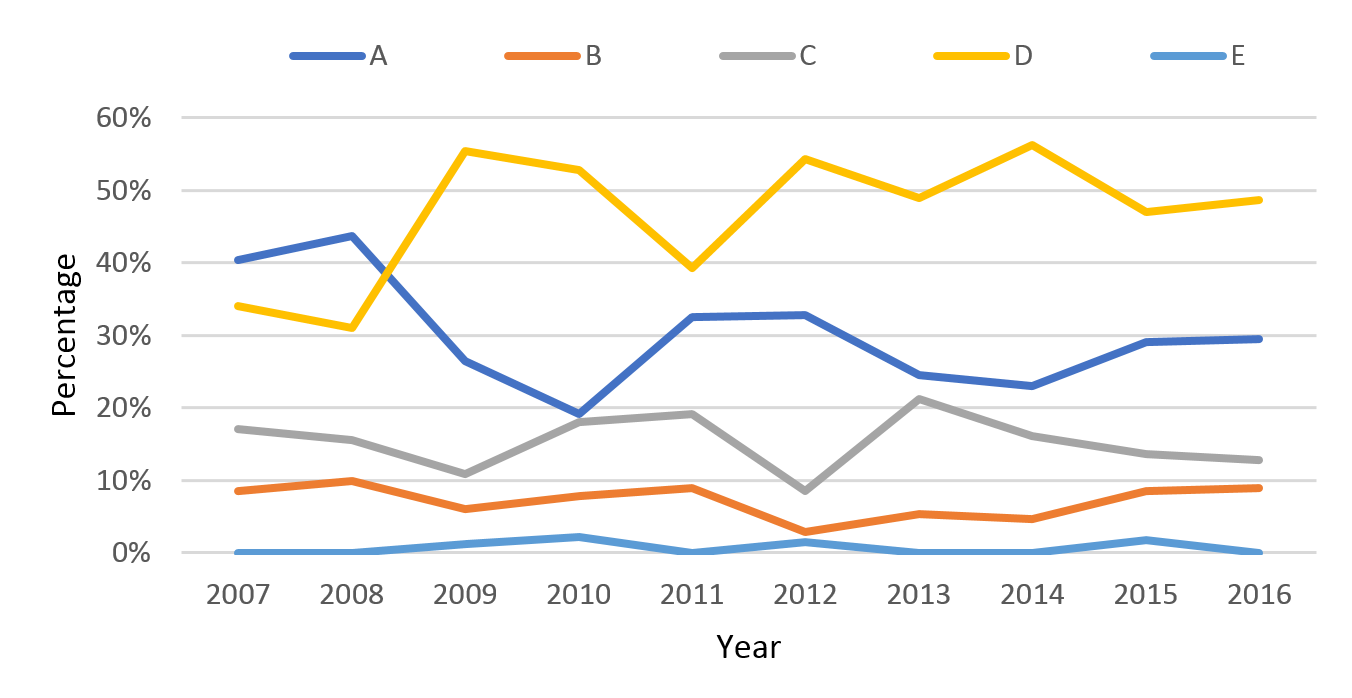
Figure 10: Age stratified AIS score on discharge.
Discharge destination
The number of patients discharged home following rehabilitation steadily declined over the study period from a high of 88% in 2008 to 77% in 2016. Patients being discharged to their own home was less common in older adults (only 51% for people aged >75). However, the rates of patients discharged home did not differ across ethnicities (New Zealand European (78%), Māori (78%) and Pacific Islander (86%)).
Discussion
This study is the largest and most comprehensive report to date on the epidemiology and demographic trends of TSCI in New Zealand patients.5,6 The mean annual incidence of TSCI in New Zealand over the 10-year period is 22 (range 17–30) per million people. This rate is lower than previously reported (49 per million people in 1998) but is consistent with the current estimated global-incident rate of 23 per million.5,6,12
The demographic of TSCI in New Zealand has changed over the last 10 years. While TSCI in men (34 per million) is three times more frequent than women (11 per million), the incidence is increasing faster in women (9% per year) than for men (6% per year). Epidemiological studies of TSCI in Japan have also noted a reduced male predominance, with the male to female ratio reducing from 4:1 in 1990, to 2:1 more recently (2004–2013).15 Over the 10-year study period the mean age of patients sustaining a TSCI increased from 43 to 48 years. Concordantly incidence rates in the older age groups showed the biggest change, increasing 11% and 10% per year in the 55–74 and >75 age groups respectively.
Over the 10-year study period the leading causes of TSCI were falls (32%), transport (32%) and sports (22%). Comparatively, in 1993 the reported leading causes of TSCI in New Zealand were transport (54%), falls (24%) and sports (11%).5,12 The pattern of decreasing transport-related TSCI and increasing number of TSCI from falls is consistent with other developed countries.2,4,12,14,15 Age influences aetiology, with falls more common in older adults (the cause for 70% of patients over the age of 75), while transport remains the most frequent mechanism in younger (16–30 years) cohorts.
A 2016 systemic review identified New Zealand as having the third highest proportion of TSCI as a result of sports injuries worldwide (behind Russia and Fiji) based on a rate of 20% reported in 1993.5,17 This study’s recorded proportion of TSCI due to sports injuries of 22% remains high and would maintain New Zealand’s standing in this comparison. Globally, rugby is responsible for 23% of all sporting-related TSCI and its popularity in New Zealand has been suggested as the reason for the high rates of sporting-related TSCI in this country.5 In 1993, Dixon et al5 reported rugby caused 74% of sporting TSCI in New Zealand.5 In comparison we identified team ball sports (including rugby) to be responsible for 20% of all sports-related TSCI in New Zealand over the recent 10-year period.17 Internationally, diving is the most prevalent cause of sports-related TSCI (35%)17, however this was not the case in our New Zealand population (18%). Water-related incidents caused 4.6% of all TSCI in New Zealand, a rate almost half that of Australia (9.0%).12 While the global rate of TSCI related to cycling is recorded as 5.5%, we found wheeled non-motorsport (including cycling and mountain biking) represented 18% of sporting-related TSCI in New Zealand.17 A recent study has shown cycling to be increasingly popular in New Zealand with hospital admissions from cycling injuries increasing 17% per year from 2012–2016.19
Cervical TSCI was the most common level of injury, occurring in 54% of patients. This level of injury is more common in older adults, with 62% of patients over 60 having a cervical TSCI compared to 47% of patients aged between 16–30 years. This is consistent with other global studies, including a Canadian study that found the proportion of cervical injuries was 88% in people >75 years, versus 46% in patients aged <35 years.2 Our study found cervical injuries were more common in Pacific Islanders (76%) and Māori (61%) compared to New Zealand Europeans (50%). The higher incidence of cervical TSCI may be due to anatomical differences in the spine, with Māori having 1mm and Pacific Islanders having 2mm smaller cervical canal than New Zealand Europeans.20 Overall, the majority of patients received surgery for their TSCI (77%). The highest proportion of patients who had surgery were in the younger age groups (89% in under 30 years), consistent with recent Canadian studies.1,2 Low-energy incomplete (particularly AIS D) cervical TSCI’s are often managed non-operatively and the probable reason for the lower surgical rates in the older age groups (66% in >75 years).1,2
One key finding of the study was that rates of TSCI in Māori were 1.8 times greater than New Zealand Europeans and increasing at a faster rate than both New Zealand Europeans and Pacific Islanders. Further work is needed to understand the basis for this disproportionate representation in Māori, given Pacific Islanders have a lower rate of injury despite also having smaller cervical canals than New Zealand Europeans.
Demographic information from this study maybe useful to identify preventative strategies aimed at reducing the numbers of TSCI. While we did not stratify aetiology data by gender, the increasing rates in both older adults and female patients are possibly explained by an increasing rate of trauma in older adult women from low energy falls resulting in incomplete cervical TSCI (eg, central cord syndrome), a trend noted worldwide.2,4,13–15 While the reason for increasing falls in older adults is multifactorial, most falls happen at home. Therefore, programmes to raise awareness and improve home safety and design are important to consider in future health resource allocation.4 In addition, a focus on cycle safety may need to be a priority in New Zealand.
There seems to have been significant progress in reducing the number of rugby and transport-related TSCI. “RugbySmart” was introduced in New Zealand by Accident Compensation Corporation (ACC) and New Zealand Rugby Union (NZRU) in 2001, which focused on educating rugby participants about physical conditioning, injury management and safe techniques in the contact phases of rugby. This coincided with a reduction in the rate of SCI arising from scrums in rugby union.21 In 2007, new rugby scrum laws were implemented, which have been implicated in the reduced rate of rugby-related TSCI in South Africa17 and may partly explain the reduced rate in our study. Further analysis of the cohort of people injured playing rugby is planned. The reduction in transport-related TSCI is postulated to be a result of improved car safety (seatbelts, air bags etc), improved roading and more awareness/advice on road safety.2,4,15 Of note, the New Zealand Ministry of Transport reports a 41% decrease in both morbidity and 50% reduction in mortality from motor vehicle crashes from 1993 to 2016.18
The strengths of this study include the large patient numbers and the 10-year timeframe. However, the majority of data were retrospectively gathered and thus limited by the extent and quality of data initially recorded and recovered. The prospective collection of NZSCIR data from 2016 onwards will negate these issues in the future. The data in this study may underestimate the true figures as some TSCI patients in New Zealand, specifically patients with minor deficits, are not always referred for SCI rehabilitation.
This study represents the first significant epidemiology publication based on information from the recently established NZSCIR. These insights help health providers understand our current patient population and have highlighted areas where further analysis may identify opportunities to improve outcomes. It has initiated further studies on the over-representation of Māori patients in this cohort and the changing status of rugby-related injuries. It directs health professionals and funders to anticipate a future where older adults will make up an increasing proportion of patients requiring treatment and rehabilitation. Collecting identical data to our parent Canadian-wide registry provides a unique opportunity to continually compare demographics and treatment practices on a country-wide basis with a respected and established international leader in SCI research.
Conclusion
This study provides the most current, extensive and accurate epidemiology review of TSCI in New Zealand and has identified important demographic trends in this patient cohort. The incidence of TSCI is increasing particularly in older adults, women and Māori. Falls are the most common cause of TSCI particularly in older adults, while cervical TSCI are the most common level of injury, with higher rates in Māori and Pacific Islanders. We confirmed a high proportion of sporting-related TSCI compared to other countries, with the highest rates in team ball sports (eg, rugby), individual water sports (eg, diving) and wheeled non-motorsports (eg, cycling/mountain biking). Despite previously recorded high rates of rugby-related TSCI in New Zealand, these have moderated. Future New Zealand public health resourcing and prevention strategies need to be cognisant of the trends demonstrated.
Aim
To investigate the epidemiology of traumatic spinal cord injury (TSCI) in New Zealand over a 10-year period.
Methods
Ambispective data of all new patients admitted to New Zealand’s two spinal rehabilitation units between January 2007 and December 2016 (n=929) were collated. Variables assessed included age at injury, gender, ethnicity, date of injury, aetiology, length of hospital stay, injury level, neurological status on discharge and discharge destination.
Results
The incidence of TSCI averaged 22 (95% CI 21–24) per million, increasing 6% a year. The average incidence for Māori (29 per million people (95% CI 25–34)) was 1.8 times higher than New Zealand European (16 per million people (95% CI 15–18)), and show an increase of 14% a year. The median age of TSCI increased from 43 to 48 years. Overall, falls (32%), transport (32%) and sports (22%) were the most common causes of TSCI. Cervical TSCI (54%) were most common, particularly in older adults (70% over 75 years) and Māori (61%) and Pacific Island (72%) patients. Surgical rates remained stable (77%) but length of stay in hospital decreased over the study period.
Conclusion
The demographic of TSCI is changing in New Zealand. The median age of patients is increasing, as is the incidence, particularly for women, older adults and Māori patients.
Authors
John Mitchell, Principal Investigator, Orthopaedic Registrar, Middlemore Hospital; Joanne Nunnerley, Co Investigator, Research Supervisor and Coordinator, Research Fellow/Academy Director, University of Otago, Christchurch; Burwood Academy of Independent Living; Chris Frampton, Co Investigator, Statistician, Professor Biostatistics, University of Otago, Christchurch; Tracey Croot, Co Investigator, Burwood Coordinator, NZ Spinal Cord Injury Registry; Alpesh Patel, Research Supervisor and Coordinator; Orthopaedic Consultant, Middlemore Hospital; Rowan Schouten, Research Supervisor and Coordinator, Orthopaedic Consultant, Christchurch Hospital.Acknowledgements
The authors thank the New Zealand Spinal Cord Injury Registry Governance Group, and the Canterbury and Counties Manukau District Health Boards. Funding for NZSCIR is provided through Accident Compensation Corporation, Canterbury and Counties Manukau District Health Boards. The NZSCIR is modelled on the Canadian Rick Hansen Spinal Cord Injury Registry (RHSCIR) in collaboration with the Praxis Spinal Cord Institute. We would like to thank Burwood Academy of Independent Living (BAIL) for coordinating our research assistants, Courtney Poole and Suzanne Reiser, who worked tirelessly inputting retrospective data, and for funding from Canterbury Orthopaedic Services (COS).Correspondence
John Mitchell, Principal Investigator, Orthopaedic Registrar, Middlemore Hospital, 21 Seine Rd, Forrest Hill, Auckland.Correspondence email
jmit068@aucklanduni.ac.nzCompeting interests
Mrs Croot is one of two NZ Spinal Cord Injury Registry (NZSCIR) Coordinators. She is involved with consenting participants, data collection, data entry, registry oversight and maintenance, as well as data access requests for the NZSCIR. She was not involved in the data extraction for this paper. Dr Nunnerley reports funding from Canterbury Orthopaedic Services during the conduct of the study.1. Pickett G, Campos-Benitez M, Keller J, Duggal N. Epidemiology of traumatic spinal cord injury in Canada. Spine. 2006; 31(7):799–805.
2. Lenehan B, Street J, Kwon B, et al. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine. 2012; 37(4):321–329.
3. Kumar R, Lim J, Mekary R, et al. Traumatic spinal injury: Global epidemiology and worldwide volume. World Neurosurgery. 2018; 113:345–e363.
4. Beck B, Cameron P, A Braaf, S, et al. Traumatic spinal cord injury in Victoria, 2007–2016. Med. J. Aust. 2019; 210:360–366.
5. Dixon G, Danesh J, Caradoc-Davies T. Epidemiology of spinal cord injury in New Zealand. Neuroepidemiology. 1993; 12(2):88–95.
6. Derrett S, Beaver C, Sullivan M, et al. Traumatic and non-traumatic spinal cord impairment in New Zealand: incidence and characteristics of people admitted to spinal units. Injury Prevention. 2012; 18(5):343–346.
7. Howard-Brown C, Civil I. New Zealand Spinal Cord Registry: a new milestone. NZ Med J. 2016 15; 129(1438):6–7.
8. Noonan VK, Kwon BK, Soril, L, et al. The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry.
9. Croot T and Young L. New Zealand Spinal Cord Injury Registry Protocol. 2019:v2.6.
10. Biering-Sørensen F, DeVivo M, Charlifue S, et al. International Spinal Cord Injury Core Data Set (version 2.0) - including standardization of reporting. Spinal Cord, 2017; 55(8):759–764.
11. ASIA Impairment Scale and the ISNCSCI Motor and Sensory Exam. (2019). http://scireproject.com/outcome-measures/outcome-measure-tool/american-spinal-injury-association-impairment-scale-ais-international-standards-for-neurological-classification-of-spinal-cord-injury/#1467983894080-2c29ca8d-88af
12. Lee B, Cripps R, Fitzharris M, et al. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014; 52(2):110–116.
13. Kehoe A, Smith J, Edwards A, et al. The changing face of major trauma in the UK. Emerg Med J. 2015; 32(12):911–915.
14. Beck B, Cameron P, Lowthian J, et al. Major trauma in older persons. BJS Open. 2018; 23(5):310–318.
15. Tafida M, Wagatsuma Y, Ma E, et al. Descriptive epidemiology of traumatic spinal injury in Japan. Journal of Orthopaedic Science. 2018; 23(2):273–276.
16. Isles S, Christey G, Civil I, Hicks P. The New Zealand Major Trauma Registry: the foundation for a data-driven approach in a contemporary trauma system. NZ Med J. 2017 Oct 6; 130(1463):19–27.
17. Chan C, Eng J, Tator C, Krassioukov A. Epidemiology of sport-related spinal cord injuries: A systematic review. The Journal of Spinal Cord Medicine. 2016; 39(3):255–264.
18. Transport.govt.nz. Road crash statistics | Ministry of Transport. [online] Available at: http://www.transport.govt.nz/mot-resources/road-safety-resources/roadcrashstatistics/
19. Singh N, Joe N, Amey A, et al. Cycling-related injuries and cycling promotion: a trauma service perspective. NZ Med J. 2019; 132(1494):41–48.
20. Faraj S, Coldham GJ, Doyle A, Baber P. Ethnic variation in cervical spine diameter. Orthopaedic Proceedings. 2015; 88-B:No.SUPP_II
21. Quarrie KL, Gianotti SM, Hopkins WG, Hume PA. Effect of nationwide injury prevention programme on serious spinal injuries in New Zealand rugby union: ecological study BMJ 2007; 334:1150.
