VIEWPOINT
Vol. 133 No. 1509 |
Healthcare-associated Staphylococcus aureus bacteraemia: time to reduce the harm caused by a largely preventable event
Staphylococcus aureus disease is associated with significant morbidity and mortality.
Full article available to subscribers
Staphylococcus aureus disease is associated with significant morbidity and mortality.1 Several studies from Australia and New Zealand looking at outcomes for S. aureus bacteraemia (SAB) across all ages have shown a 30-day all-cause mortality of 20%; highest in the older age group.2–5 Over the last 30 years in New Zealand, there has been a relentless increase in the incidence of skin and soft tissue infection caused by S. aureus, largely driven by an increase in community-associated methicillin-susceptible S. aureus infections.6–8
Healthcare-associated S. aureus bacteraemia (HA-SAB) is defined as an episode of S. aureus bacteraemia occurring 48 hours after hospitalisation (not present or incubating on admission) or occurs in the context of an indwelling medical device, within 30 days of surgery (or 90 days of surgery involving implantable devices), within 48 hours of a related invasive instrumentation or incision or is associated with neutropaenia associated with cytotoxic therapy. The onset of infection for 60–70% of all SAB episodes occurs in the community, but about half of these infections are associated with recent hospitalisation, surgery or a procedure, or associated with a medical device; most commonly a vascular access device.4 The more common sources of HA-SAB include vascular access devices, surgical site infections, lower respiratory tract infections and skin and soft tissue infections.9–11 Patients who develop infections often have a longer length of stay, may die and often require further interventions contributing to increased healthcare costs.12
There is significant ethnic disparity associated with the burden of S. aureus disease in New Zealand; the incidence of both invasive and non-invasive S. aureus disease, even after adjusting for socioeconomic deprivation, is highest among Māori and Pacific Peoples. In particular, Māori are three times more likely and Pacific Peoples are five times more likely than non-Māori and non-Pacific peoples to have S. aureus skin and soft tissue infections.6,7 For Māori and Pacific Peoples the rates of SAB are two and four times that of European New Zealanders, respectively.2,3
To quote Florence Nightingale “The very first requirement in a hospital is that it should do the sick no harm”. We agree with this sentiment. Reporting of district health board (DHB) hospital-acquired bloodstream infections (BSI) and HA-SAB rates has been considered an important indicator of quality of care for over 20 years, but little consideration has been given to implementing interventions known to reduce the rate of either. A significant proportion of healthcare-associated infections, such as HA-SAB, are preventable, and to continue to report on this in the absence of any nationally-led initiatives to reduce these events is a missed opportunity. The Health Quality & Safety Commission Infection Prevention and Control programme has been effective at improving compliance with hand hygiene and reducing surgical site infections for hip and knee arthroplasties and cardiac surgery. To support our view that action needs to be taken, we have summarised the history of healthcare-associated BSI surveillance in New Zealand, and provided examples of actions taken to reduce HA-SAB rates in other jurisdictions. We then recommend the main interventions that need to be delivered at a national level to reduce the ongoing harm caused by HA-SAB.
In New Zealand, hospital-acquired BSI surveillance was started in the late 1990s to provide important national data about this serious and potentially preventable event. It was established as part of the Key Performance Indicators programme set up by the Crown Company Monitoring and Advisory Unit in 1999. Subsequently, reporting shifted to the Ministry of Health’s Hospital and Health Services Balanced Scorecard Performance Indicator Framework. The Australian Council on Healthcare Standards definition for hospital-acquired blood stream infections was adopted and episodes of BSI occurring more than 48 hours after admission to hospital were included. Within the health sector the reliability of this data was questioned because of inconsistencies in the way district health boards (DHB) defined such infections and reporting of results were not viewed as useful.13
In the early 2000s, surveillance was restricted to monitoring of HA-SAB episodes only. As with the previous surveillance programme the case definition was provided but no training or monitoring of the application of the case definition was incorporated into the programme. Both the Ministry of Health’s National Quality Improvement Programme (NQIP) hand hygiene project and the Health Quality and Safety Commission’s (the ‘Commission’) Hand Hygiene New Zealand programme have subsequently used the reported HA-SAB rate as the outcome marker for their programmes. Improvement in hand hygiene compliance was matched with improvement in the HA-SAB rate at Auckland DHB but this has not been replicated at a national level despite significant improvements in hand hygiene compliance.14,15
In 2017 the Commission assessed the accuracy of individual DHB reporting of the denominator (bed-days) for the HA-SAB rate. Analysis revealed inconsistencies in reported numbers; both under- and over-reporting of bed days so it decided that the denominator data would be obtained from the National Minimum Data Set (NMDS). The Commission developed an implementation guide to support and standardise the application of the definition, which included flow charts and a set of clinical scenarios providing guidance on how to apply the HA-SAB definition.16 Webinars were held to educate and support consistency in the application of the definition. This was in keeping with the recommendation by World Health Organization guidance on core competencies of infection prevention and control programmes that “a national training programme for performing surveillance should be established to ensure the appropriate and consistent application of national surveillance guidelines and corresponding implementation toolkits”.17
Following this work the aggregated national rate of HA-SAB has increased and remains stable at a rate of 0.13 HA-SAB per 1,000 bed days (Figure 1). The increase can be attributed to increased HA-SAB rates at several referral DHB.
Figure 1: Outcome marker, healthcare-associated Staphylococcus aureus bacteraemia per 1,000 bed days by month, March 2012–March 2019.
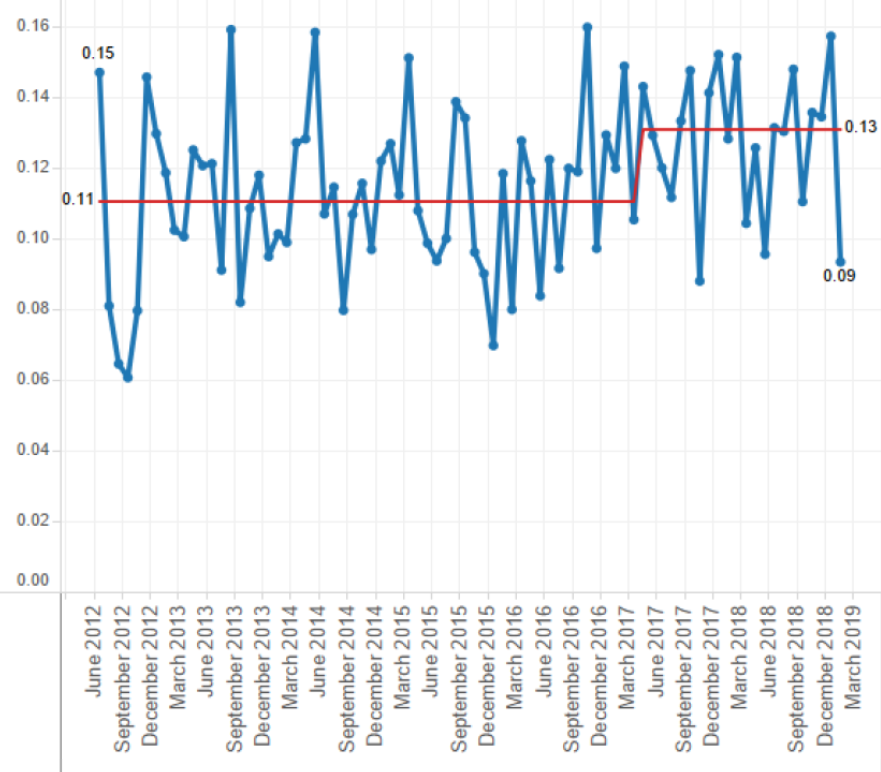
The rate of HA-SAB is used as a national performance indicator in countries such as Australia and Scotland, and as a measurement of the process of care to improve outcomes for patients with SAB by individual hospitals in other countries.18–21
In 2008 the Australian Health Ministers endorsed the reporting of HA-SAB cases occurring in public hospitals by states and territories as part of performance reporting under the National Healthcare Agreement. The performance benchmark for public HA-SAB was set at no more than 2.0 per 10,000 days of patient care for acute care public hospitals by 2011–12 in each state and territory.18 Between 2012–13 and 2015–16 the rate of hospital-associated SAB has decreased from 0.94 cases to 0.74 cases per 10,000 days of patient care. The overall rate of HA-SAB in all public hospitals in Australia for 2016–17 was 0.76 cases per 10,000 days of patient care.18
The burden of HA-SAB in Australia sits within principal referral hospitals. Principal referral hospitals provide a very broad range of highly-specialised services and have large patient volumes. These hospitals accounted for 54% of all events and for 37% of patient care under surveillance and in 2016–17 the rate ranged from 1.01–1.23 HA-SAB cases per 10,000 days of patient care. Unlike Australia, there has been no subset analysis of New Zealand HA-SAB events based on hospital size or complexity to determine where the burden of diseases is located.
Despite similarities between the definition used for determining the rate of HA-SAB in Australia and HA-SAB in New Zealand the two rates are not comparable for a number of reasons. Firstly, a significant proportion of hospital beds in Australia are within the private sector; in the 2016–17 reporting period a total of 89 private hospitals reported HA-SAB data accounting for only 14.1% of all known private hospitals.18 The national HA-SAB rate in private hospitals who had reported data was lower than the national benchmark at 0.38 cases per 10,000 days of patient care. Secondly, patients in Australia can move between privately and publicly funded care with limited linkage of the care provided. In contrast, in New Zealand there are a limited number of private surgical beds, and in situations where complications arise the patient is often transferred to or re-admitted to a DHB hospital. And thirdly, New Zealanders have a national health index number and this allows individuals to be uniquely identified for the purposes of treatment and care, and for maintaining medical records in DHB hospitals. This supports sharing of information between DHB healthcare providers about events such as HA-SAB.
Healthcare-associated infections were nominated as a priority area by the Australian Commission for Safety and Quality in Health Care (ACSQHC) and a range of national and local initiatives have been established to reduce the occurrence with leadership provided by ACSQHC. These initiatives include a national hand hygiene improvement programme, Hand Hygiene Australia, national infection control guidance which includes information on managing medical devices such as vascular access devices and urinary catheters, building capacity to address skill and knowledge gaps, an antimicrobial stewardship initiative and a national surveillance initiative to monitor healthcare-associated infections.
Likewise, in 2010 when the Commission was established the infection prevention and control programme was one of the first programmes implemented. This led to the reinvigoration of the Hand Hygiene New Zealand programme, a national initiative to reduce central line associated blood stream infections in intensive care and high dependency units in New Zealand, Target CLAB Zero, and the establishment of a national surgical site infection improvement programme.23–25
The recent work to improve the consistency of the application of the HA-SAB definition has resulted in a more accurate outcome measure to monitor improvement in response to these quality improvement initiatives. Hand hygiene plays a significant role in reducing the transmission of bacteria between patients and surfaces within a healthcare setting. The Hand Hygiene Australia programme has shown a reduction in HA-SAB in Australian hospitals eight years after implementation.26 Hospitals where a number of improvement interventions have been implemented have shown sustained reductions in methicillin-resistant SAB.27 It should be acknowledged that improving hand hygiene compliance alone is not the only activity associated with a reduction in HA-SAB rates. Unpublished New Zealand data indicates that about 50% of all HA-SAB are associated with vascular access devices, and 20% are associated with surgery or other procedures (personal communication, N. Grae). About 15% have no clear source. Interventions shown to improve the adherence to best practice for the insertion and maintenance of central and peripheral vascular access devices are associated with reduced HA-SAB.28,29 More recently the Commission has piloted a perioperative staphylococcal decolonisation bundle, termed ‘the anti-staph bundle’, to reduce the risk of S. aureus surgical site infections in patients undergoing cardiac surgery and hip and knee arthroplasties. Preliminary results look promising (personal communication, N. Grae) and are in keeping with the research showing that anti-staph bundles reduce S. aureus infections.30
In conjunction with sustaining hand hygiene compliance other quality improvement initiatives targeting interventions known to reduce HA-SAB are required. The major areas for improvement in process are the use of vascular access devices; especially long-term central vascular devices and peripheral intravascular catheters (PIC). The latter devices are in common use; nearly half of all adult inpatients at Auckland District Health Board have a PIC, of which 20% had no apparent clinical indication (personal communication, S. Muttaiyah). Implementation of the ‘anti-staph bundle’ as part of the perioperative care for a wider range of surgical procedures and across all DHBs should also be considered. This intervention has been shown to reduce SSI rates and is cost-effective.31
Māori carry an unacceptable burden of S. aureus disease and we need to work in partnership with Māori to reduce this inequity both at a secondary and primary healthcare level.6–8 Māori children have higher rates of colonisation with S. aureus and as a consequence, higher rates of skin and soft tissue infection.32 There is limited knowledge about the rate of S. aureus colonisation in adults in New Zealand. One study of mostly young people (age range 15–24) showed that 18% had nasal colonisation with S. aureus but the study had a number of limitations.33 We argue that adults residing in households with children colonised with S. aureus may also have higher rates of S. aureus colonisation increasing their risk of healthcare-associated S. aureus infection should they require admission. Colonisation with S. aureus increases the risk of surgical site infections three- to ten-fold.34 A better understanding of the S. aureus colonisation across all age and ethnic groups is required to better inform prevention strategies.
To reduce the burden of healthcare-associated infections caused by S. aureus in New Zealand there needs to be a commitment at a national level to implement interventions aimed at reducing them. A collaborative quality improvement approach should be taken to share expertise and experiences across all DHBs and the private surgical hospital sector; the production of guidelines alone will not be effective. National programmes such as ‘Target CLAB Zero’ and the SSII programme have shown reduced harm to patients and similar initiatives to reduce peripheral vascular access device infections are needed. These programmes should be linked across primary, secondary and tertiary care and be co-designed with consumers to increase the likelihood of success. In conjunction there needs to be an increased focus on improving skin care in young New Zealanders, particularly young Māori and Pacific children. In healthcare settings the most obvious areas to target include the practices of inserting and maintaining vascular access devices and reducing skin colonisation with S. aureus prior to any surgery.
Reducing the rates of HA-SAB is crucial to improve the outcomes for all accessing healthcare in New Zealand. No more need for counting: time for action.
See more related
Authors
Sally Roberts, Microbiology, LabPlus, Auckland District Health Board, Auckland; Nikki Grae, Infection Prevention and Control Programme, Health Quality and Safety Commission, Wellington; Sharmini Muttaiyah, Microbiology, Auckland District Health Board, Auckland; Arthur J Morris, Microbiology, Auckland City Hospital, Auckland.Correspondence
Dr Sally Roberts, Microbiology, LabPlus, Auckland District Health Board, Auckland.Correspondence email
sallyrob@adhb.govt.nzCompeting interests
Nil.1. Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015; 28:603–660.
2. Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Int Med J. 2001; 31:97–103.
3. Hill PC, Wong CGS, Voss LM, et al. Prospective study of 125 cases of Staphylococcus aureus bacteraemia in children in New Zealand. Pediatr Infect Dis J. 2001; 20:868–73.
4. Turnidge JD, Kotsanas D, Munckhof W, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2009; 191:368–73.
5. McMullan BJ, Bowen A, Blyth CC, et al. Epidemiology and mortality of Staphylococcus aureus bacteraemia in Australian and New Zealand children. JAMA Pediatr. 2016; 170:979–86.
6. O’Sullivan CE, Baker MG, Zhang J. Increasing hospitalisations for serious skin infections in New Zealand children, 1990–2007. Epidemiol Infect. 2011; 139:1794–804.
7. Williamson DA, Zhang J, Ritchie SR, Roberts SA, Fraser JD. Baker MG. Staphylococcus aureus in New Zealand, 2000–2011. Emerg Infect Dis. 2014; 20:1156–61.
8. Williamson DA, Lim A, Thomas MG, et al. Incidence, trends and demographics of Staphylococcus aureus infections in Auckland, New Zealand, 2001–2011. BMC Infect Dis. 2013; 13:569.
9. Bishara J, Goldberg E, Leibovici L, Samra Z, Shaked H, Mansur N, Pail M. Healthcare-associated vs. hospital-acquired Staphylococcus aureus bacteremia. Int J Infect Dis. 2012; 16:e457–463
10. Collignon P, Cruikshank M, Dreimanis D. Staphylococcus aureus bloodstream infections: an important indicator for infection control. Healthcare Infect. 2009; 14:165–71.
11. Worth L, Spellman T, Bull A, et al. Staphylococcus aureus bloodstream infection in Australian hospitals: findings from a Victorian surveillance system. Med J Aust. 2014; 200:282–4.
12. van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteraemia. Clin Microbiol Rev. 2012; 25:362–86.
13. Report of the Controller and Auditor-General. Management of Hospital-Acquired Infection. Office of the Auditor-General New Zealand, Vol 1. June 2003 ISBN 0-478-18105-1.
14. Roberts SA, Sieczkowski C, Campbell T, Balla G, Keenan A. Implementing and sustaining hand hygiene culture change programme at ADHB. NZ Med J. 2012; 125(1354):75–85.
15. Freeman JT, Dawson L, Jowitt DM, et al. The impact of the Hand Hygiene New Zealand programme on hand hygiene practices in New Zealand public hospitals. NZ Med J 2016; 129(1443):67–76.
16. Health Quality & Safety Commission New Zealand. Implementation guide for the surveillance of Staphylococcus aureus bacteraemia (SAB). August 2017 Accessed 2nd July 2019 http://www.hqsc.govt.nz/assets/Infection-Prevention/Hand-Hygiene/PR/HHNZ_HA_SAB_Implementation_Guide.pdf
17. Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level. Geneva: World Health Organisation; 2016. Licence: CC BY-NC-SA.3.0 IGO
18. Australian Institute of Health and Welfare 2017. Staphylococcus aureus bacteraemia in Australian public hospitals 2016–17: Australian hospital statistics. Health services series no. 83. Cat. No. HSE 198. Canberra AIHW ISBN 978-1-76054-272-6.
19. Murdoch F, Danial J, Morris AK, et al. The Scottish enhanced Staphylococcus aureus bacteraemia surveillance programme: the first 18 months of data in adults. J Hosp Infect. 2017; 97:133–9.
20. Dendle C, Martin RD, Cameron DR, et al. Staphylococcus aureus bacteraemia as a quality indicator for hospital infection control. Med J Aust. 2009; 191:389–92.
21. Rosa R, Wawrzyniak A, Sfeir M, Smith L, Abbo LM. Performance of care and outcomes in patients with Staphylococcus aureus bacteraemia. J Hosp Med. 2016; 11:27–32.
22. Lam JC, Gregson DB, Robinson S, et al. Infectious diseases consultation improves key performance metrics in the management of Staphylococcus aureus bacteraemia: a multicentre cohort study. JAMMI 2019; 4:24–32.
23. Gray J, Proudfoot S, Power M, Bennett B, Wells S, Seddon M. Target CLAB Zero: a national improvement collaborative to reduce central line-associated bacteraemia in New Zealand intensive care units. NZ Med J 2015; 128(1421):13–21.
24. Morris AJ, Panting AL, Roberts SA, Shuker C, Merry AF. A new surgical site infection improvement programme for New Zealand: early progress. NZ Med J 2015; 128(1414):51–9.
25. Morris AJ, Roberts SA, Grae N, Hamblin R, Shuker C, Merry AF. The New Zealand Surgical Site Infection Improvement Programme: a national quality improvement programme reducing orthopaedic surgical site infections. NZ Med J 2018; 131(1479):45–56.
26. Grayson ML, Stewardson AJ, Russo PL, et al, Effects of the Australian National hand Hygiene Initiative after 8 years on infection control practices, healthcare worker education, and clinical outcomes: a longitudinal study. Lancet Infect Dis. 2018; 18:1269–77.
27. Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. Eight years of decreased methicillin-resistant Staphylococcus aureus healthcare-associated infections associated with a Veterans Affairs prevention initiative. Am J Infect Control. 2017; 45:13–6.
28. Kok J, O’Sullivan MV, Gilbert GL. Feedback to clinicians on preventable factors can reduce hospital onset Staphylococcus aureus bacteraemia rates. J Hosp Infect. 2011; 79:108–14.
29. Blauw M, Foxman B, Wu J, Rey J, Kothari N, Malani AN. Risk factors and outcomes associated with hospital-onset peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia. OFID 2019; 6:1–6.
30. Ma N, Morris AJ, Grae N, Roberts SA, Systematic review of a patient care bundle in reducing staphylococcal infections in cardiac and orthopaedic surgery. ANZ J Surg 2017; 87:239–46.
31. Rennert-May E, Conly J, Smith S, et al. A cost-effective analysis of mupirocin and chlorhexidine gluconate for Staphylococcus aureus decolonization prior to hip and knee arthroplasty in Alberta Canada compared to standard of care. Antimicrob Res Infect Control 2019; 8:113.
32. Hobbs MR, Grant CC, Thomas MG, et al. Staphylococcus aureus colonisation and its relationship with skin and soft tissue infection in New Zealand Children. Eur J Clin Microbiol Infect Dis 2018; 37:2001–10.
33. Best N, Fraser JD, Rainey PB, Roberts SA, Thomas MG, Ritchie SR. Nasal carriage of Staphylococcus aureus in healthy Aucklanders. NZ Med J 2011; 124(1332):31–9.
34. Sakir A, Brégeon F, Mège JL, Rolain JM, Blin O. Staphylococcus aureus nasal colonisation: an update on mechanisms, epidemiology, risk factors and subsequent infections. Front Microbiol. 2018; 9:2419.
