ARTICLE
Vol. 133 No. 1510 |
Fasting prior to cardiac catheterisation: a single-centre observational study
Angiography is a common procedure, undertaken in almost 20,000 patients in New Zealand in 2017.
Full article available to subscribers
Angiography is a common procedure, undertaken in almost 20,000 patients in New Zealand in 2017.1 In the early days of cardiac catheterisation, available contrast agents frequently caused nausea and vomiting, with an associated risk of aspiration pneumonitis. Fasting patients for angiography, except in the emergency setting, became standard practice with recommendations that patients be fasted for four to six hours prior to procedures despite the lack of robust evidence to support this.2 Angiograms undertaken with modern contrast media have low complication rates.3 Observational studies in patients undergoing emergency angiograms for ST elevation myocardial infarction show an extremely low risk of aspiration even in high-risk groups requiring intubation.4
Delays and rescheduled procedures because patients are insufficiently fasted have a large impact on patients and the optimal allocation of limited health resources. Anecdotal data suggests that patients are fasted for long periods as angiography lists are rejigged to accommodate emergencies or unexpectedly long cases. Prolonged fasting can increase the risk of contrast-induced nephropathy and hypoglycaemia in vulnerable patients.3,5,6
This prospective study was undertaken to describe the duration and effects of fasting practices in patients undergoing elective coronary angiography at Auckland City Hospital.
Methods
A prospective, observational study was undertaken of consecutive patients undergoing elective coronary angiography and percutaneous coronary intervention (PCI) at a single institution, Auckland City Hospital, over a period of six months. All patients who underwent elective coronary angiography and PCI between 7 August 2017 and 7 February 2018 were included in this study.
Ethical and institutional approval was obtained prior to the study commencing. The study was approved as low risk, with individual informed consent not required. Patients were instead given the choice to opt out of data collection up to a month following their procedure. For outpatients, study information sheets were sent out two weeks before the scheduled procedure together with the patient’s appointment letter. For hospital inpatients, a ward staff nurse gave the patient an information sheet at least one day prior to their procedure.
A worksheet collected data on baseline demographics, length of fasting and adverse events. Cardiac Investigation Unit staff nurses were trained to complete these forms and data was collected from the time of commencement to completion of the angiogram.
Data on the procedure, including contrast volume, was collected from the catheterisation report.
The primary endpoint was the frequency of adverse events associated with fasting such as hunger, hyper/hypotension, nausea, vomiting and aspiration. Secondary endpoints were the duration of fasting, frequency of patient-reported outcomes such as thirst, sore throat, disorientation and dry mouth and the use of anti-emetic medication.
Definitions
Hypertension was defined as blood pressure above 140/90mmhg7 and hypotension below 90/60mmhg.8Hyperglycaemia was defined as capillary blood sugar on finger-stick above 11mmol/L9 and hypoglycaemia below 4mmol/L.10
The renal association criteria (The UK eCKD Guide) were used to define chronic kidney disease.11
Data was entered into a Microsoft Excel database. Statistical analysis used Statistical Analysis System (SAS) including Student T-Test and ANOVA for continuous data measurement. Results are presented as number (%) or mean (standard deviation) if parametric and median (interquartile range) of non-parametric.
Results
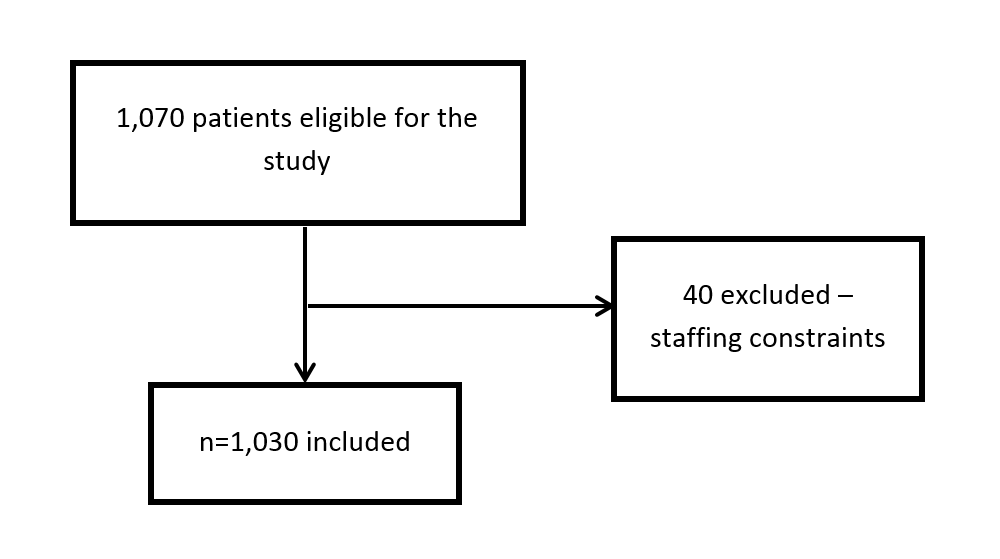
A total of 1,070 elective cases were performed over six months, of which 1,030 were included in this study—40 patients were not included as staff were too busy to complete checklists (Figure 1). No patients opted out of data collection.
Figure 1: Study flow.
Participant characteristics
The mean age of participants was 66±11.9 years and 68% were male (Table 1). The ethnic distribution is reflective of the Auckland and Northland population with 66% European and 14% Māori participants. Cardiovascular risk factors were present in the majority of patients with hypertension the most frequent (72%). Renal dysfunction, defined as an eGFR <60ml/min/1.73m2 was present in 23%. The majority of study participants underwent elective coronary angiography only (69%).
Table 1: Patient characteristics.
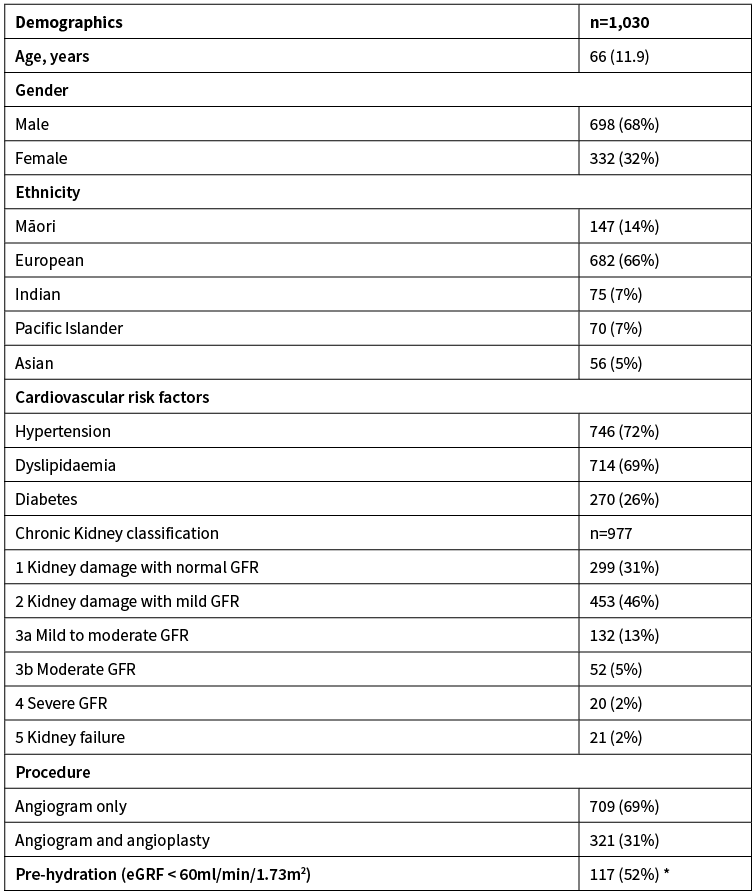
*Percentage meant to be pre-hydrated. Results are presented as mean (SD) or number (%).
Length of fasting
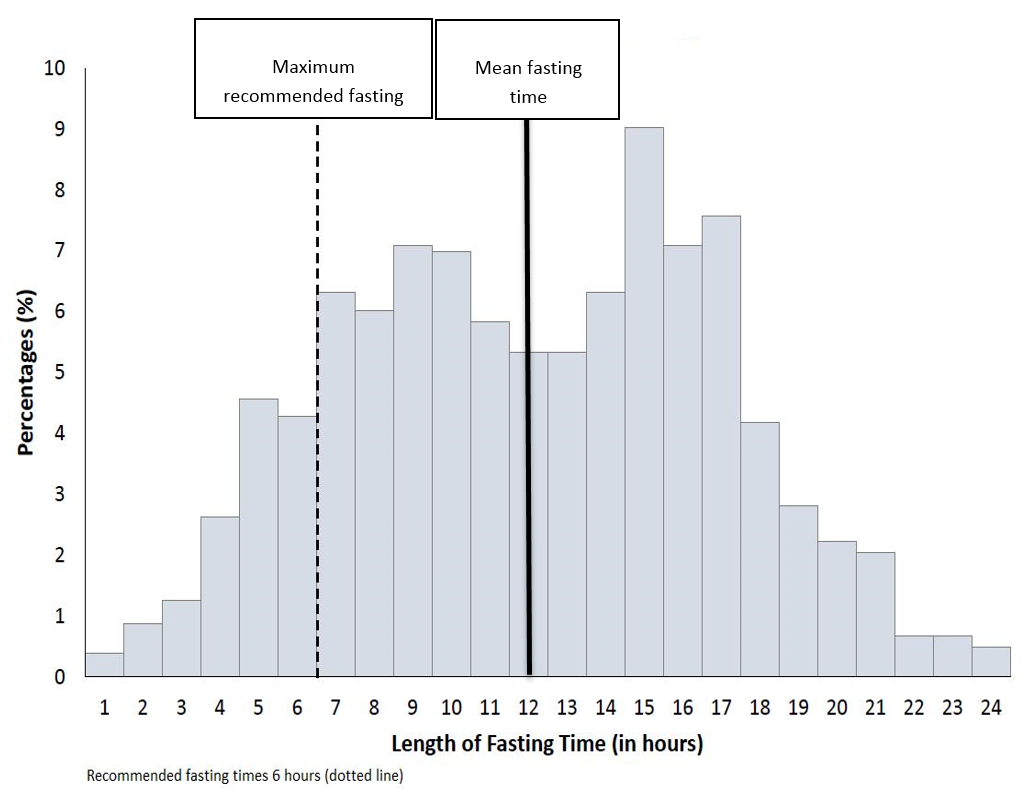
The mean length of fasting was 11.6 hours (+4.9) with 80% of patients (n=821) fasting longer than recommended (Figure 2).
Figure 2: Length of fasting in hours.
Complications
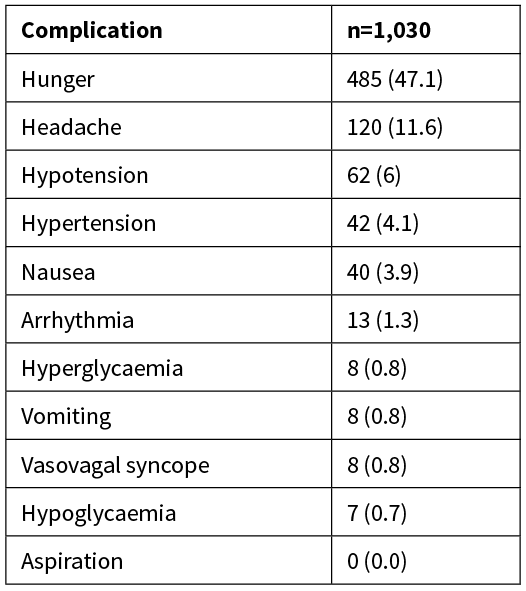
The most common complication was hunger at 47.1% (Table 2), while headache occurred in 11.6% of study participants, and nausea and vomiting in 3.9% and 0.8% respectively. Of the 40 patients who experienced nausea, 26 required anti-emetic drugs. Hypotension was recorded in 6% of patients, hypertension in 4.1% and hyperglycaemia in 0.8%. Hypoglycaemia (0.7%) and vasovagal syncope (0.8%) were uncommon. There were no reports of aspiration.
Table 2: Complications of fasting presented as number (%).
Of the 225 classified as having renal dysfunction, 108 (48%) were not pre-hydrated as per guidelines and catheter laboratory protocols.
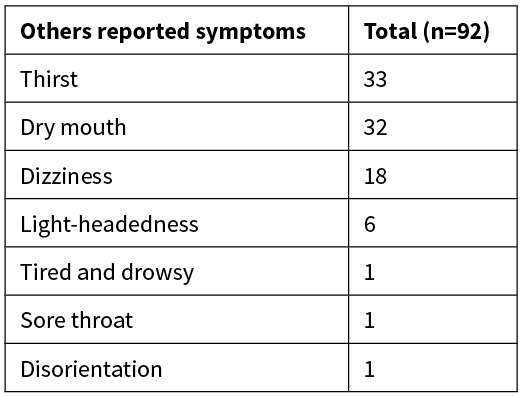
Other patient-reported outcomes are listed in Table 3.
Table 3: Other patient-reported outcomes presented as number.
Discussion
This study found that in this cohort, the incidence of clinically important complications was low, but the average length of fasting was significantly longer than guideline recommendations.
Despite recommendations that patients are fasted three to six hours for solid food and up to two hours for clear liquids pre-procedure,2,12,13 80% of patients included in this study were fasted longer than recommended. Reasons for this are complex, but may include the dynamic changes to angiogram lists based on urgency and complexity of cases leading to delays.
Hospital and national guidelines are based on extrapolations from anaesthetic studies showing that fasting reduces aspiration pneumonia in patients undergoing general anaesthetic.14 However, there is little published data to support fasting prior to angiogram, a procedure usually undertaken with conscious sedation.2,12,13 The largest observational study to date, which included 1,916 non-fasted patients, had no cases of aspiration pneumonia.15 Moreover, patients with ST elevation myocardial infarctions have angiograms without fasting with few adverse outcomes.15,16 Risk of vomiting and aspiration are low even in the setting of emergency coronary artery bypass graft surgery following angiography (0.1%).4,17 Joint guidelines from the Royal College of Anaesthetists and Royal College of Emergency Medicine recommended no fasting for minimal sedation.18
The population enrolled in this study were relatively healthy adult participants without the increased risk of aspiration pneumonia, similar to other studies.19 Specific risk factors common in patients undergoing angiogram, such as older age, renal insufficiency and diabetes mellitus, can increase the risk for complications of fasting. The risk of the dehydration includes electrolyte disturbances20 and contrast-induced nephropathy.5 This is especially important in those who have chronic kidney disease.3,5 Hydration (orally or intravenously) is key to reducing acute kidney injury in those with chronic kidney disease. Protocols are in place to identify and pre-hydrate those at risk of contrast-induced nephropathy. However, we found that almost half of patients with renal dysfunction were not pre-hydrated, increasing the risk of acute kidney injury.
Those with diabetes are particularly disadvantaged by prolonged fasting for two reasons; diabetes medications need to be taken with food and fasting increases the risk of hypoglycaemia. A number of patients had hyperglycaemia due to missed medications and worryingly, some developed hypoglycaemia.
The most common complaints from patients related to discomfort due to hunger and thirst. This finding is in agreement with a review in the perioperative setting, which showed significantly higher hunger and thirst scores in patients who were fasted compared to patients who received water, coffee and carbohydrate drinks.19 This may not be medically important, but can impact significantly on the patient experience. Elderly patients in particular do not tolerate these symptoms well and this can negatively affect their wellbeing.
Strengths and limitations
This study was undertaken in a single centre and may not be generalisable to other cardiac catheterisation units. However, there were few exclusion criteria and patients were enrolled sequentially, suggesting this was representative of the normal patient cohort.
Data was collected by different staff nurses who may have had a different interpretation of the outcome variables. This was mitigated by training all staff on data collection prior to the study start and having detailed descriptions of predefined endpoints available.
There were challenges determining the exact duration of fasting. Patients were considered to have stopped fasting at the end of their procedure. This was a pragmatic decision as the actual time resumption of food intake occurred was not recorded. Actual total fasting duration may be longer than that reported.
The most important limitation was that no data was collected to reliably assess rates of contrast-induced nephropathy as the majority of patients were discharged home post-procedure, without further laboratory values being obtained. Further research is needed to better assess the impact of fasting on development of acute kidney injury.
Conclusion
This study found that patients were fasted for longer than recommended and that despite guidelines recommending its use, pre-hydration was underutilised in patients at high risk of contrast-induced nephropathy. There were no episodes of aspiration found. Further studies are required to determine whether there is any role for fasting patients prior to non-emergency cardiac catheterisation.
Aim
Previous generation contrast agents were associated with high rates of nausea, vomiting and risk of aspiration leading to recommendations to fast prior to the procedure. However, modern contrast agents are well tolerated with a low risk of aspiration. Our current guidelines recommend fasting four to six hours before elective and semi-urgent cardiac catheterisation despite a lack of evidence to support this. We sought to determine the duration and effects of fasting at our centre.
Methods
A single-centre prospective observational study in patients undergoing elective cardiac catheterisation over a six-month period between 7 August 2017 to 7 February 2018 at Auckland City Hospital, New Zealand.
Results
One thousand and thirty patients with a mean age of 66±12 years underwent catheterisation. Sixty-seven percent were male, 26% had diabetes, 72% had hypertension and 23% had stage 3 or worse chronic kidney disease. The mean duration of fasting was 11.6±4.9 hours with 80% fasting longer than recommended. One hundred and eight (48%) patients with documented chronic kidney disease did not receive recommended pre-hydration. The most common symptoms related to fasting were hunger (47 %), nausea (3.9%) and vomiting (0.8%). Hypertension (4.1%) and hyperglycaemia (0.8%) occurred due to missed medication. There were no reports of aspiration.
Conclusion
Most patients were fasted for significantly longer than recommended and pre-hydration was underutilised in patients at high risk of contrast-induced nephropathy. There were no episodes of aspiration with modern contrast agents. Further studies are required to evaluate the need for fasting prior to non-emergency cardiac catheterisation.
Authors
Sheila Badana Bacus, CIU/ CVRU, Auckland District Health Board, Auckland; John Parsons, School of Nursing, University of Auckland, Auckland; Jocelyne Benatar, Cardiology Department, Auckland City Hospital, Auckland; Jithendra Somaratne, CIU/ CVRU, Green Lane Clinical Centre, Auckland City Hospital, Auckland; Mark Webster, Green Lane Cardiovascular Service, Auckland City Hospital, Auckland; Rachael Parke, School of Nursing, University of Auckland, Auckland.Correspondence
Mrs Sheila Badana Bacus, CIU/ CVRU, Auckland District Health Board, 9 Park Road Grafton, Auckland 1024.Correspondence email
sheilabad04@yahoo.comCompeting interests
Nil.1. Kerr A, Williams MJ, White H, et al. The All New Zealand Acute Coronary Syndrome Quality Improvement Programme: Implementation, Methodology and Cohorts (ANZACS-QI 9). The New Zealand medical journal. 2016;129(1439):23-36.
2. Naidu SS, Rao SV, Blankenship J, et al. Clinical expert consensus statement on best practices in the cardiac catheterization laboratory: Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012;80(3):456-64.
3. Andreucci M, Faga T, Pisani A, et al. Acute kidney injury by radiographic contrast media: pathogenesis and prevention. BioMed research international. 2014;2014:362725.
4. Grayson AD, Moore RK, Jackson M, et al. Multivariate prediction of major adverse cardiac events after 9914 percutaneous coronary interventions in the north west of England. Heart. 2006;92(5):658-63.
5. Hiremath S, Akbari A, Shabana W, et al. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous? A systematic review of the evidence. PLoS One. 2013;8(3):e60009.
6. Chipps D, Wong, V., Ross, G., & Wong, J. Peri-operative diabetes management guidelines. Australian Diabetes Society. Retrieved on October 29, 2019 from https://diabetessocietycomau/documents/perioperativediabetesmanagementguidelinesfinalcleanjuly2012pdf ( ).
7. High Blood Pressure (Hypertension). Retrieved from https://wwwmayoclinic.org/disease-conditions/high-blood-pressure/symptoms-causes/syc-20373410 Mayo Clinic; 2018a [cited 2019 October 29].
8. Low blood pressure (hypotension). Retrieved from https://www.mayoclinic.org/diseases-conditions/low-blood-pressure/symptoms-causes/syc-20355465 Mayo Clinic2018b [cited 2019 October 29].
9. Managing Diabetes (Diabetes and Hyperglycemia). Retrived from https://www.diabetes.co.uk/Diabetes-and-Hyperglycaemia.html Diabetes Co UK 2018a [cited 2019 October 29].
10. Managing Diabetes (Diabetes and Hyperglycemia). Retrieved from https://www.diabetes.co.uk/diabetes-and-hypoglycaemia.html Diabetes Co UK2018b [cited 2019 October 29].
11. The UK eCKD Guide. Retrived from https://renal.org /information–resources /the-uk-eckd-guide/ckd-stages/ Renal Association2017 [cited 2019 October 29].
12. Bashore TM, Balter S, Barac A, et al. 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update: A report of the American College of Cardiology Foundation Task Force on Expert Consensus documents developed in collaboration with the Society of Thoracic Surgeons and Society for Vascular Medicine. J Am Coll Cardiol. 2012;59(24):2221-305.
13. Thomas SP, Thakkar J, Kovoor P, et al. CSANZ Position Statement on Sedation for Cardiovascular Procedures (2014). Heart, lung & circulation. 2015;24(11):1041-8.
14. Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. 2017;126(3):376-93.
15. Hamid T, Aleem Q, Lau Y, et al. Pre-procedural fasting for coronary interventions: is it time to change practice? Heart. 2014;100(8):658-61.
16. Rolley J, Kuhn L, Berry D, et al. Pre-procedural fasting for patients undergoing percutaneous coronary interventions: Preliminary results from a multi-centre retrospective audit. Heart, Lung and Circulation. 2015;24:S294.
17. Warner MA, Caplan, R. A., Epstein, B. S., Gibbs, C. P., Keller, C. E., Leak, J. A., & Weinlander, C. M. Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures. Survey of Anesthesiology. 2000;44(1):47-48.
18. Lloyd G. Procedural sedation in emergency medicine. RCEM learning. Retrieved from https://www.rcemlearning.co.uk/reference/ procedural-sedation-emergency-medicine-2018/ 2018 [cited 2019 October 29].
19. Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003(4):Cd004423.
20. Iggulden H. Dehydration and electrolyte disturbance. Nursing standard (Royal College of Nursing (Great Britain) : 1987). 1999;13(19):48-54; quiz 55-7.
