ARTICLE
Vol. 133 No. 1510 |
Intensive care unit utilisation post-oesophagectomy
Worldwide, oesophageal cancer is the sixth most common cancer, and the seventh most common cause of cancer-related mortality—responsible for 1 in 20 cancer deaths in 2018.
Full article available to subscribers
Worldwide, oesophageal cancer is the sixth most common cancer, and the seventh most common cause of cancer-related mortality—responsible for 1 in 20 cancer deaths in 2018.1 Surgical resection remains the mainstay of treatment for localised oesophageal adenocarcinoma; however, oesophagectomy is a complex surgical procedure with high reported incidence of morbidity (59%) and perioperative mortality (4.5% 90-day mortality).2 As a result, post-operative care traditionally includes an intensive care unit (ICU) admission. This is currently routine practice for all patients undergoing oesophagectomy in Christchurch Public Hospital, New Zealand; however, due to ICU capacity constraints, elective cases are not infrequently cancelled on the day of surgery. This poses considerable inconvenience and psychological difficulty for the patient and their family, and poses logistical challenges for the surgical team who need to operate in a timely fashion post-neoadjuvant therapy.
In the era of enhanced recovery and with refined anaesthetic and surgical approaches, including early extubation and improved analgesic regimes, our experience was that these patients required minimal ICU-level care and were usually discharged to lower-level care day one post-operatively. If these patients could safely be managed in a non-ICU setting this would circumvent the above mentioned difficulties and could also potentially result in cost saving. The purpose of this audit, therefore, was to examine the utilisation of ICU-level intervention post-oesophagectomy to investigate feasibility and safety of a non-ICU based care post-operative package.
Methods
Study setting
Christchurch Public Hospital is the largest tertiary-level hospital in the South Island of New Zealand, serving a population of approximately 558,830 people. It is one of six specialist oncological hospitals in New Zealand appointed by the New Zealand Government. On average there are 12 oesophagectomies performed per year. Five surgeons performed the oesophagectomies in the study period. There is no formal Enhanced Recovery after Surgery (ERAS) protocol for oesophagectomy; however, the general principles of ERAS are followed. There is no standalone high dependency unit (HDU), and ICU has a high proportion of ventilated patients; therefore, the effective HDU space is low. The highest level of care available outside ICU is in the surgical progressive care unit (SPCU), which has a 1:2 nursing ratio; however, there is no capacity for inotropic support, positive pressure or invasive respiratory support.
Data collection
All patients who underwent an oesophagectomy at Christchurch Public Hospital between 1 January 2015 and 31 May 2019 were identified from electronic database scOPe®. Data were collected from a review of paper and electronic records. This included demographic details, ASA status, length of ICU stay, requirement for ventilatory and inotropic support, inpatient deaths and length of hospital stay. Complications were defined as per the Esophagectomy Complications Consensus Group (ECCG)3 where possible—limitations exist due to retrospective nature of collection and variation in record keeping standards.
Ethics
The need for the formal approval for the research was waived by the National (New Zealand) Health and Disability Ethics Committee given the provisions of the study being a clinical audit. Locality approval was granted by the Canterbury District Health Board (CDHB)—ref 19189.
Results
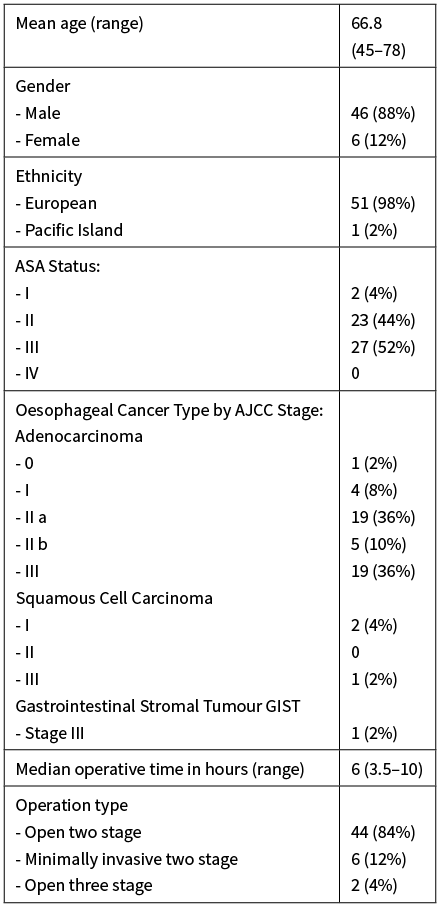
Between January 2015 and May 2019, 52 patients underwent oesophagectomy in Christchurch Hospital. Demographic characteristics, ASA status, staging and operative details are presented in Table 1. The indication for resection was adenocarcinoma in the majority of cases (92%), while three patients with squamous cell carcinoma and one with an obstructing gastrointestinal stromal tumour underwent resection. Most procedures were open two-stage thoracoabdominal oesophagectomy (84%). Six patients had synchronous multi-organ resection: one small bowel resection for incidental finding of small bowel GIST, three splenectomies for intraoperative injury, one cholecystectomy and one lung lobectomy.
Table 1: Demographic, pathological and operative details.
Post-operative data
All 52 patients were admitted to ICU post-operatively. ICU interventions are detailed in Table 2. The median intensive care stay was 23 hours. Thirty-nine patients (75%) were extubated prior to arrival on the intensive care unit, with a further six (12%) extubated that same day in the ICU. Fourteen patients (29%) had hypoxaemia, which required more than face mask or simple nasal prong oxygen—10 of these were managed with high flow nasal prongs, three required CPAP and one patient required BiPAP (one of these routinely used CPAP for obstructive sleep apnoea). Five patients required re-intubation.
Table 2: ICU interventions.
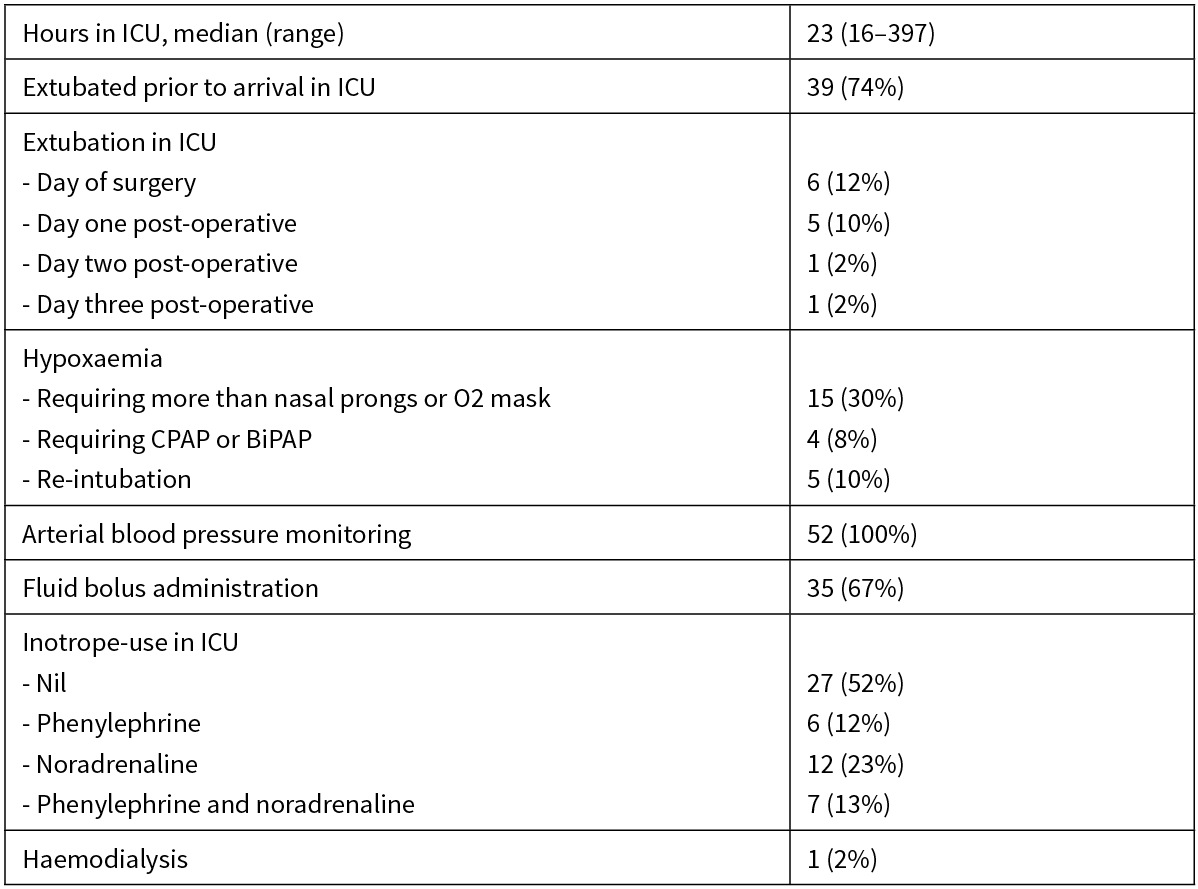
All 52 patients had continuous intra-arterial blood pressure monitoring as part of standard monitoring in ICU. Thirty-five patients (67%) were administered fluid boluses on the ICU for either hypotension or low urine output. Twenty-seven patients (52%) did not require any inotropic support while in the ICU. Only one patient required haemodialysis in the ICU for acute renal failure.
Of those who did not require any ICU-level support in the immediate post-operative ICU admission, the median age was 68.3, 48% were ASA II, 52% were ASA III and mean operative time was 5.7 hours. For those who did require ICU level intervention, the median age was 65.7, 7% were ASA I, 38% were ASA II, 55% were ASA III and mean operative time was 6.6 hours.
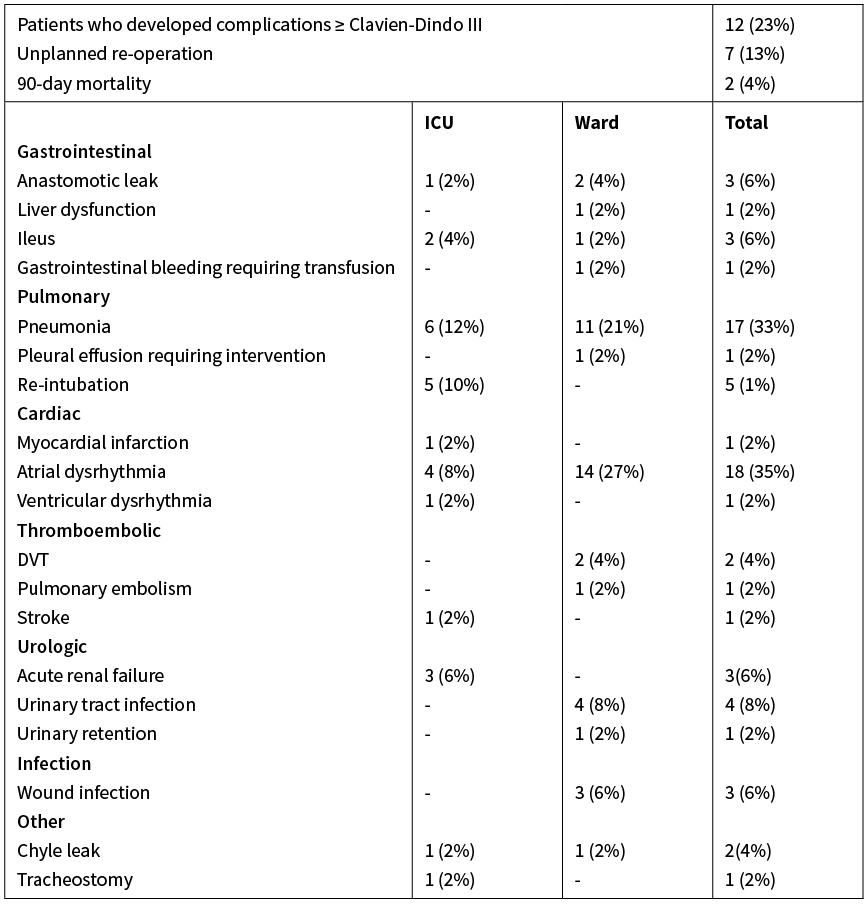
Eight patients (16%) required re-admission to ICU following complications while on either the SPCU or the general surgical ward. The median total hospital stay was 12 days (range 4–38).
Post-operative complications are presented in Table 3. The most common complication was atrial fibrillation, and this occurred predominantly in the SPCU or ward setting. Seventeen patients (32%) developed hospital-acquired pneumonia post-operatively. Seven patients (13%) required reoperation to resolve major post-operative complications and there were two inpatient deaths. One patient died day 13 from sepsis and multi-organ failure due to an anastomotic leak. The second died day 16 from sepsis and multi-organ failure following chyle leak and hospital-acquired pneumonia. No other patients died within 90 days post-operatively.
Table 3: Post-operative complications.
Discussion
We have conducted a retrospective audit of the use of ICU-specific resources post-oesophagectomy in a tertiary-level hospital in New Zealand. This demonstrates that a large proportion of patients could safely be managed outside of ICU, with possible scope for cost saving and reduced uncertainty around surgical planning associated with day of surgery cancellation related to ICU capacity.
Three quarters of the patients were extubated prior to admission to ICU, in keeping with recent published Australasian data.4 The rate of readmission to ICU was low and complication rates compare well to international data, and to historical reported rates from this institution.2,5
Worldwide there is large variation in the post-operative care protocols following oesophagectomy. A large retrospective study looked at 7,878 patients undergoing oesophagectomy across 162 hospitals in the US.6 Overall 65% were cared for in ICU post-operatively; however, there was a large range (3.6–100%), showing the wide variability between hospitals. The overall hospital mortality in that study was 6.9% and there was a 43.7% complication rate. Of interest, there was no association between hospital use of ICU and mortality in this study. Robertson et al7 and Ghosh et al8 both looked at patients managed perioperatively in an HDU setting. Each study concluded that patients could be safely managed post-oesophagectomy on a surgical HDU with ICU admission rates of 16% and 16.3% respectively.
Reduction of hospital expenditure is a long-term goal worldwide, however this should not be at the expense of the patient’s quality of healthcare. While half of the patients in our study did not ultimately require ICU-level care, the remaining half did—therefore in order to achieve cost and resource saving, prospective identification of which group patients will fall into is needed. Numerous scoring systems have been developed for this purpose. A recent systematic review identified 11 such tools including POSSUM (and variations), and those developed by Steyerberg and colleagues, Ra and colleagues, and Bartels and colleagues. This review did not identify any tool that could safely be applied to clinical practice due to unreliable results, with most model predictions frequently exceeding observed outcomes. Included studies were also limited by biases and small sample sizes.9
An alternative approach, described by Ghosh et al, is a pathway in which haemodynamically stable patients were extubated, observed in the post anaesthetic care unit (PACU) and transferred to the HDU, while haemodynamically unstable patients or those unable to be extubated were transferred to the ICU.8 While Christchurch does not have a defined, standalone HDU, the SPCU could be used in this role (with appropriate equipment, staff training and accreditation). While this does not obviate the need for ICU availability pre-operatively, nor avoid potential day of surgery cancellations, it could result in improved resource efficiency and cost savings. Furthermore, knowledge of a 50% chance of a patient not requiring ICU admission may influence the multidisciplinary decision to proceed on the day of surgery.
The limitations of this study include the retrospective nature of data analysis and the bias inherent with this approach and the relatively small sample size. The strengths are the completeness of the data set.
Conclusion
This study shows a large proportion of post-operative oesophagectomy patients do not require ICU level support; however, in the absence of a reliable pre-operative predictive tool, post-operative ICU care is currently still required. An individualised approach to post-operative care could be explored whereby an assessment period in PACU is utilised to determine if ICU or SPCU is the most appropriate location for ongoing care. Such an approach would have the potential to divert up to 50% of the group away from ICU, utilising this resource more appropriately and with improved cost effectiveness.
Aim
Oesophagectomy is a complex operation, with high rates of morbidity and mortality. Traditional post-operative care often involves admission to an intensive care unit, however with advancing surgical and anaesthetic techniques this may not be routinely required. The objective of this study is to investigate the utilisation of intensive care-specific resources following oesophagectomy in a New Zealand tertiary hospital.
Methods
All patients undergoing oesophagectomy over a five-year period at Christchurch Hospital, New Zealand were identified and data collected. Utilisation of ICU-specific resources and the occurrence of complications in relation to ICU discharge were recorded.
Results
Fifty-two patients underwent oesophagectomy between 1 January 2015 and 31 May 2019. The majority (75%) were extubated prior to admission to ICU, and only 8% required non-invasive positive pressure ventilation after extubation. Haemodynamic support with inotropic or vasopressor agents was required in 48% of patients. Most complications were managed in a non-ICU setting. The ICU readmission rate was 16%—all but one of these readmissions was following reoperation.
Conclusion
This study shows a large proportion of post-operative oesophagectomy patients do not require ICU level support, however in the absence of a reliable pre-operative predictive tool, post-operative ICU care is still required in our setting. An individualised post-operative approach could be explored to help divert stable patients, potentially up to half of the group, away from ICU.
Authors
Michael O’Grady, General Surgery Registrar, Canterbury District Health Board, Christchurch; Rebecca Firth, Resident Medical Officer, Canterbury District Health Board, Christchurch; Ross Roberts, General Surgeon, Canterbury District Health Board, Christchurch.Correspondence
Michael O’Grady, Department of General Surgery, Christchurch Hospital, 2 Riccarton Avenue, Christchurch.Correspondence email
michael.ogrady@cdhb.health.nzCompeting interests
Nil.1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. Ca Cancer J Clin 2015; 65(2):87–108.
2. Low DE, Kuppusamy M, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019; 269(2):291–8.
3. Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy. Ann Surg 2015; 262(2):286–94.
4. Meng R, Bright T, Woodman RJ, Watson DI. Hospital volume versus outcome following oesophagectomy for cancer in Australia and New Zealand. Anz J Surg 2019; 89(6):683–8.
5. Beenen E, Jao W, Coulter G, Roberts R. The high volume debate in a low volume country: centralisation of oesophageal resection in New Zealand. New Zealand Medical J 2013; 126(1374):34–45.
6. Wunsch H, Gershengorn HB, Cooke CR, et al. Use of Intensive Care Services for Medicare Beneficiaries Undergoing Major Surgical Procedures. Anesthesiology 2016; 124(4):899–907.
7. Robertson S, Skipworth R, Clarke D, et al. Ventilatory and Intensive Care Requirements Following Oesophageal Resection. Ann Royal Coll Surg Engl 2006; 88(4):354–7.
8. Ghosh S, Steyn RS, Marzouk J, Collins FJ, Rajesh PB. The effectiveness of high dependency unit in the management of high risk thoracic surgical cases. Eur J Cardio-thorac 2004; 25(1):123–6.
9. Warnell I, Chincholkar M, Eccles M. Predicting perioperative mortality after oesophagectomy: a systematic review of performance and methods of multivariate models. Bja Br J Anaesth 2015; 114(1):32–43.
