ARTICLE
Vol. 133 No. 1510 |
Population-level exposures associated with MRSA and ESBL-E. coli infection across district health boards in Aotearoa New Zealand: an ecological study
Antimicrobial resistance (AMR) is a major global health threat, undermining the sustainability and safety of modern healthcare.
Full article available to subscribers
Antimicrobial resistance (AMR) is a major global health threat, undermining the sustainability and safety of modern healthcare.1,2 The importance of AMR is reflected in the World Health Organization’s Global Action Plan on Antimicrobial Resistance. The Action Plan provides a mandate and framework for member states to develop plans at a national level.2 At both the national and international level, effective AMR response plans require an understanding of the factors driving AMR development and spread.
Differences in AMR between countries have been attributed to differences in antimicrobial use, per capita income, population density and levels of corruption.3–6 Other purported causes of AMR variation include poor infection control practices in human healthcare and agricultural settings; poor sanitation; environmental contamination from pharmaceutical runoff; agricultural antibiotic use; and international travel/migration patterns.7–10 However, compared to studies identifying risk factors at the level of the individual, relatively few studies have sought to identify exposures associated with AMR at the level of the population and in particular, regional populations within a given country.
Population-level risk factors for AMR are not necessarily the sum of individual risk profiles within a population. Studies designed to identify risk factors for individuals within a population cannot identify risk factors to which members of the population are universally exposed. In addition, the transmissibility of AMR means that one individual’s risk of having a resistant organism in turn affects the risk profile of others in the population. Additional insights may therefore be gained by investigating variation in AMR rates between populations.
In light of this, we performed an ecological study to investigate two specific AMR-associated organisms in Aotearoa New Zealand; methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase producing Escherichia coli (ESBL-E. coli). In particular, we sought to gain insight into population-level exposures associated with MRSA and ESBL-E. coli infection and any differences between the two organisms.
Methods
Using an ecological study design and available national surveillance, census and survey data, we investigated the association between MRSA and ESBL-E. coli infection and a range of population-level variables among 18 geographically distinct populations defined by district health boards (DHBs). The relationships were described utilising Spearman’s (rs) correlational analysis on GraphPad Prism version 7 (GraphPad Software, CA, USA). Statistical significance was defined as p<0.05. MRSA and ESBL-E. coli infection incidence rates for 2013 were obtained from the national survey (one month) of all public and private laboratories performed by the Institute of Environmental Science and Research Limited (ESR).11,12 Isolates from a clinical site were considered to represent infection. Isolates from presumed screening sites (nasal and groin for MRSA; rectal for ESBL-E. coli) were excluded to remove bias due to differences in active surveillance practices between DHBs.
Demographic data for each DHB population, including the proportion aged <5 years and ≥65 years; the proportion reporting European, Māori, Pacific and Asian ethnicities; and the proportion who were overseas born new arrivals in New Zealand (<1 year), was obtained from New Zealand census 2013 data.13 Population socioeconomic deprivation, as assessed by the proportion of each DHB population living in the most deprived NZdep2013 index quintile, was obtained from the New Zealand Index of Deprivation Atlas.14 The NZdep2013 index is a relative measure of socioeconomic deprivation that is assigned to small geographical units nationwide. It is made up of census 2013 variables covering communication; income; employment; qualifications; home ownership; support; living space and transport.15 DHB-level household crowding data, as assessed by the proportion of each DHB population living in crowded conditions (as per Canadian National Occupancy Standard) in 2013, was obtained from a Ministry of Health publication: Analysis of Household Crowding based on Census 2013 data.16 DHB population community antimicrobial usage in defined daily doses (DDD) per 1,000 persons per day (DID) was obtained from the ESR Antimicrobial Consumption Report.17 Population variable data from two pairs of geographically adjacent DHBs (Canterbury and South Canterbury; Capital and Coast and Hutt Valley) were merged as MRSA and ESBL-E. coli infection rates from these respective pairs were not differentiated in the 2013 ESR surveys.
Results
The incidence rate of MRSA and ESBL-E. coli infection across the 18 DHB populations is illustrated in Figure 1. Co-correlation between the evaluated population-level variables, which affect interpretation of the results for MRSA and ESBL-E. coli given below, is demonstrated in Table 1.
Figure 1: Incidence rate (per 100,000 per month) of MRSA and ESBL-E. coli infection across DHB populations.
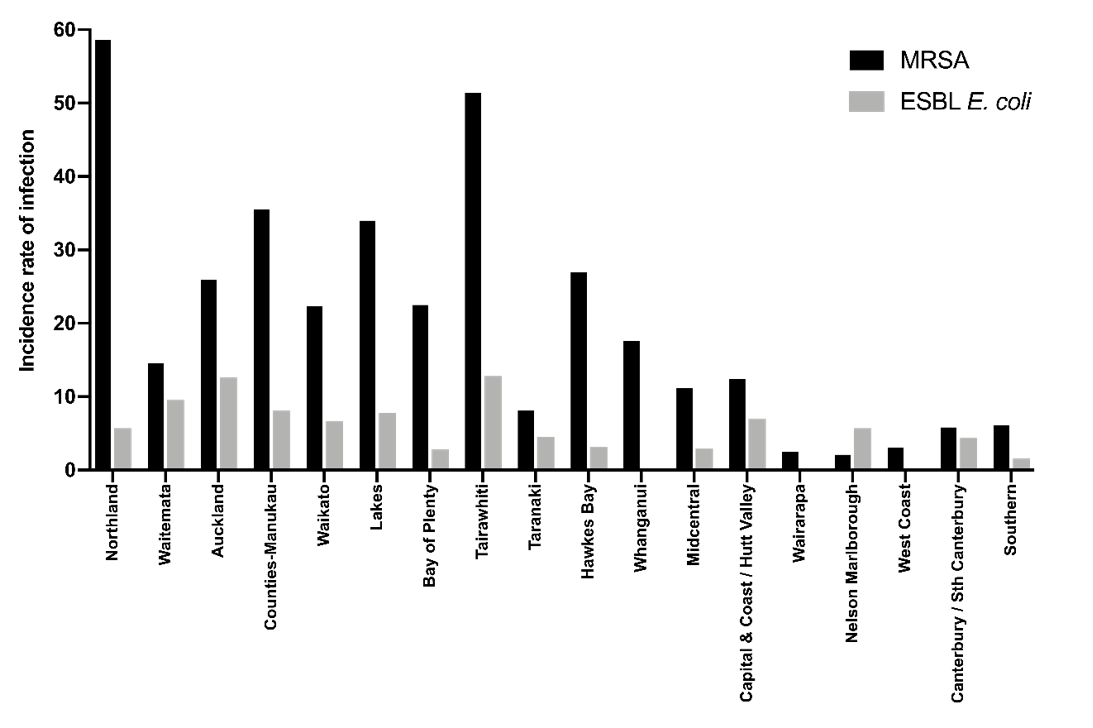
Table 1: Pairwise correlation between DHB population variables.
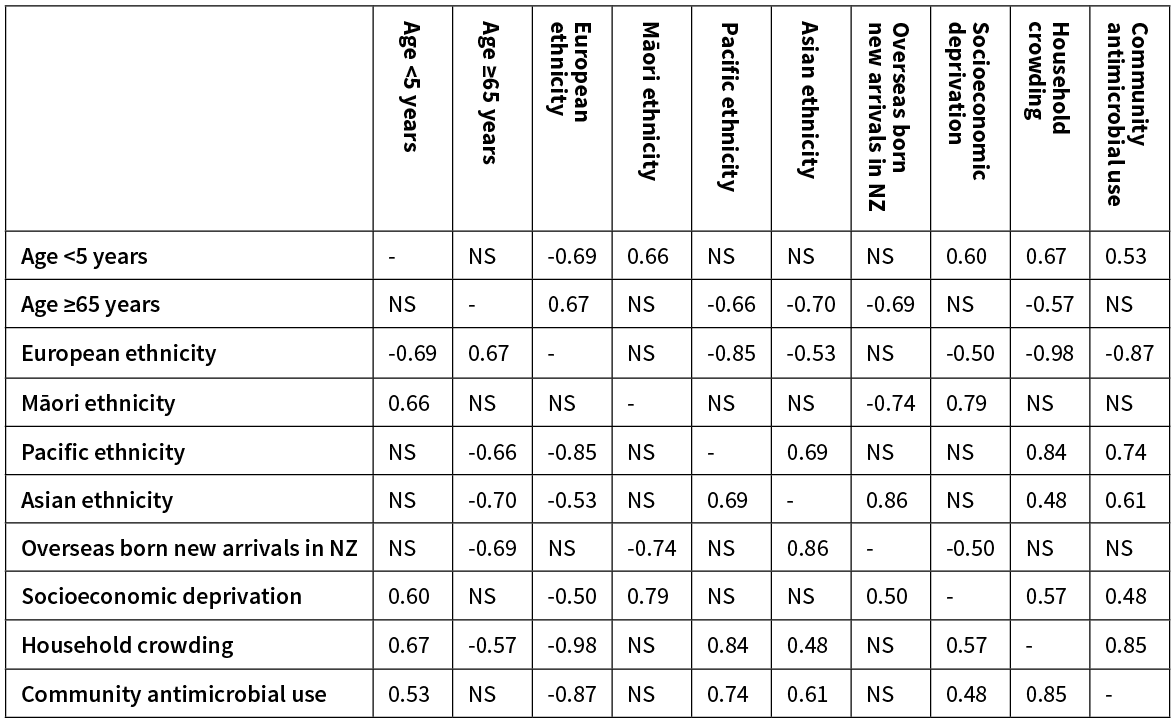
Note: rs values are given in the table when there was a significant (p<0.05) correlation between the two given population variables. NS, not significant.
The incidence of MRSA infection had a very strong positive correlation with the proportion of the population living in crowded housing conditions (Figure 2, Table 2). It also had a strong positive correlation with the proportion of the population aged <5 years; living in the most socioeconomically deprived NZdep2013 quintile; and with community antimicrobial use (Figure 2, Table 2). It had a moderate positive correlation with proportion of the population reporting Māori ethnicity and Pacific ethnicity (Figure 2, Table 2). MRSA infection had a strong negative correlation with the proportion of the population reporting European ethnicity (Figure 2, Table 2).
Table 2: DHB population variables and association with incidence of MRSA and ESBL-E. coli infection.
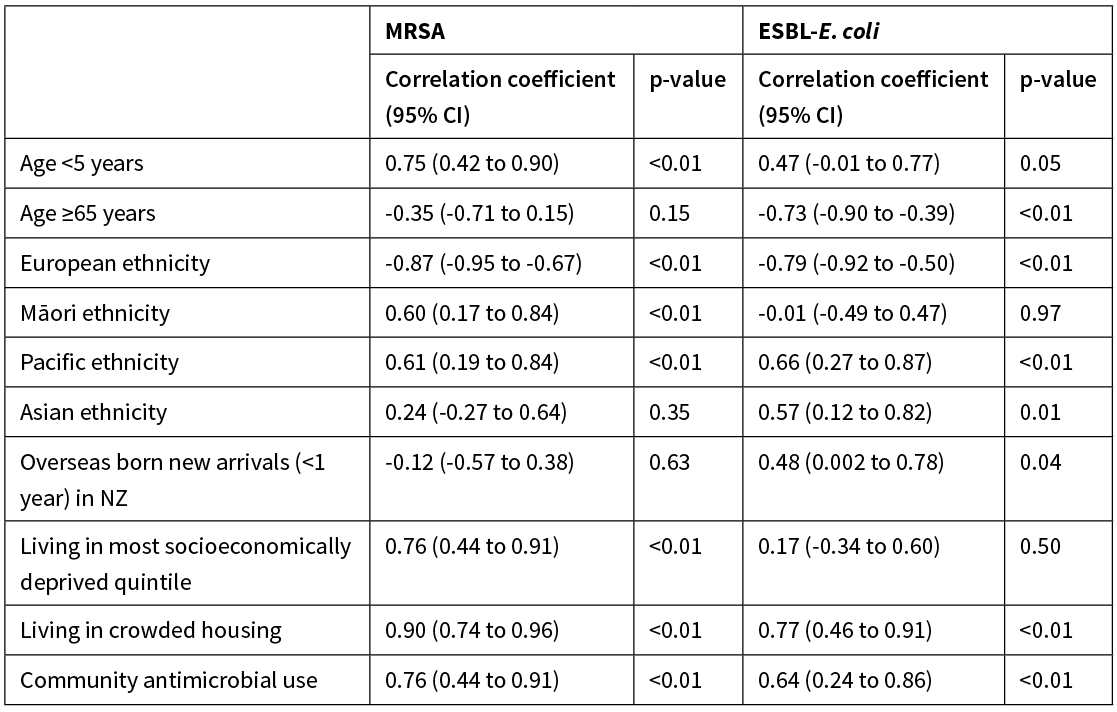
CI, confidence interval.
Figure 2: DHB population variables versus incidence of MRSA and ESBL-E. coli infection (per 100,000 per month).
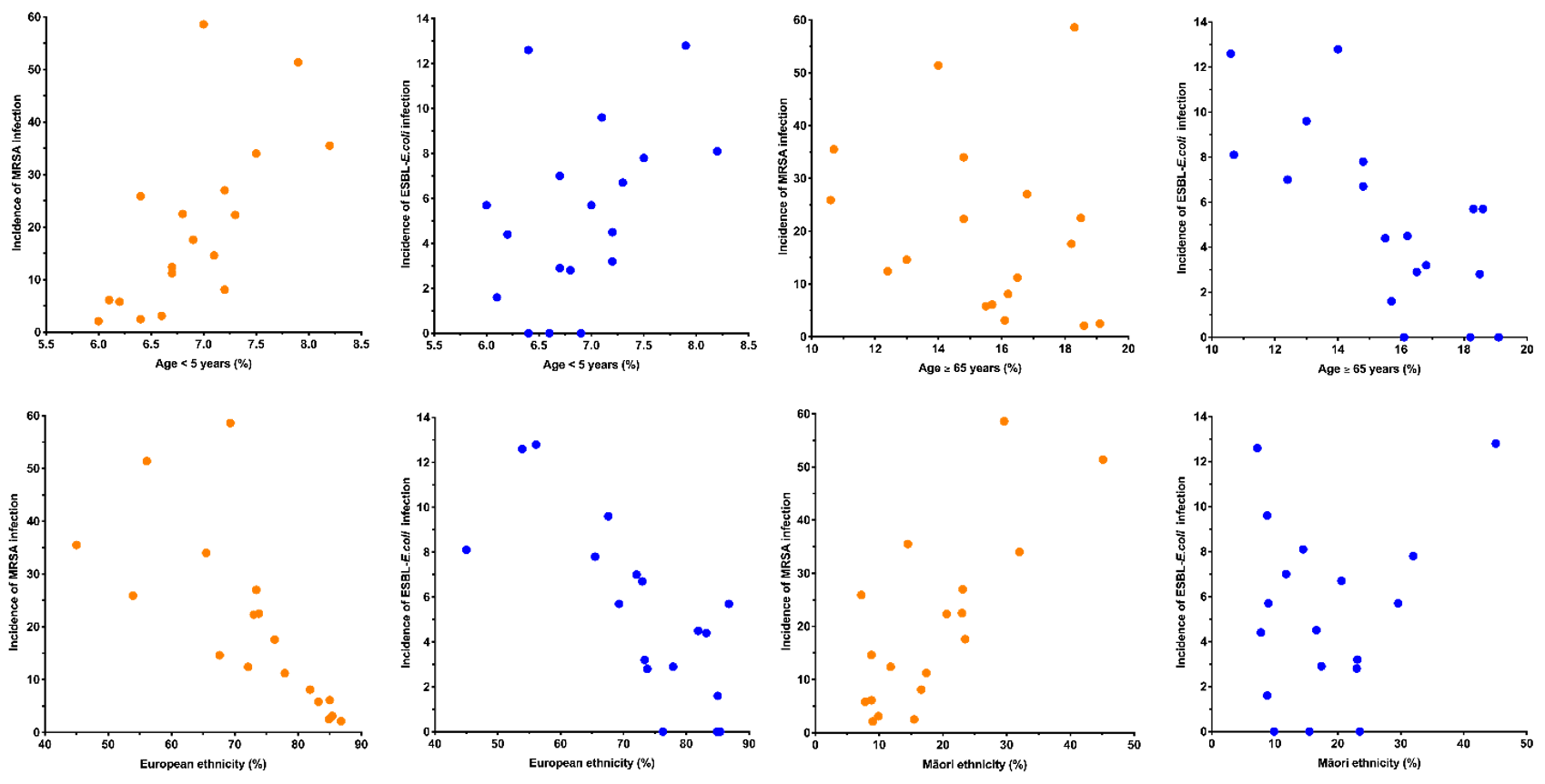
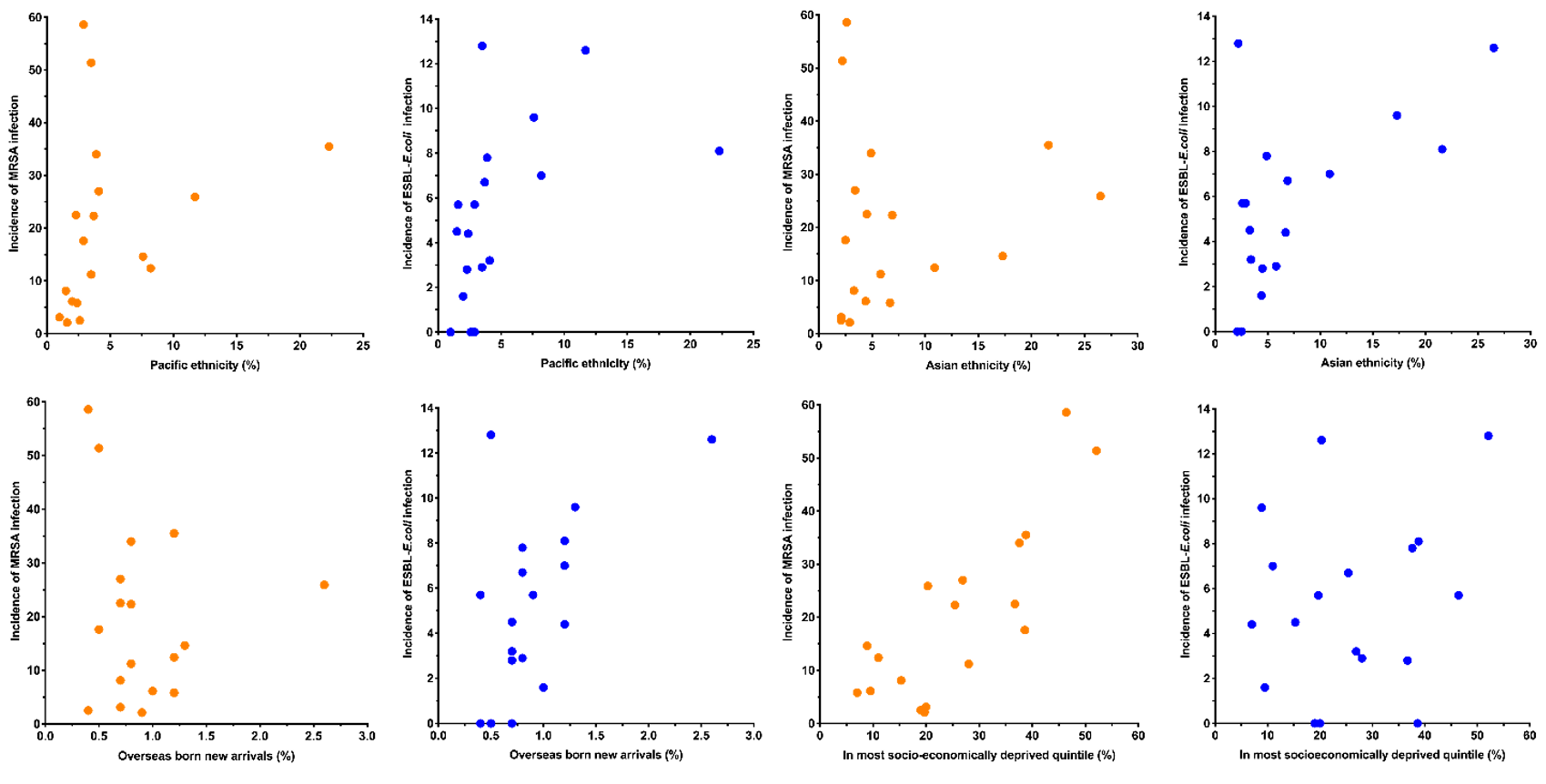
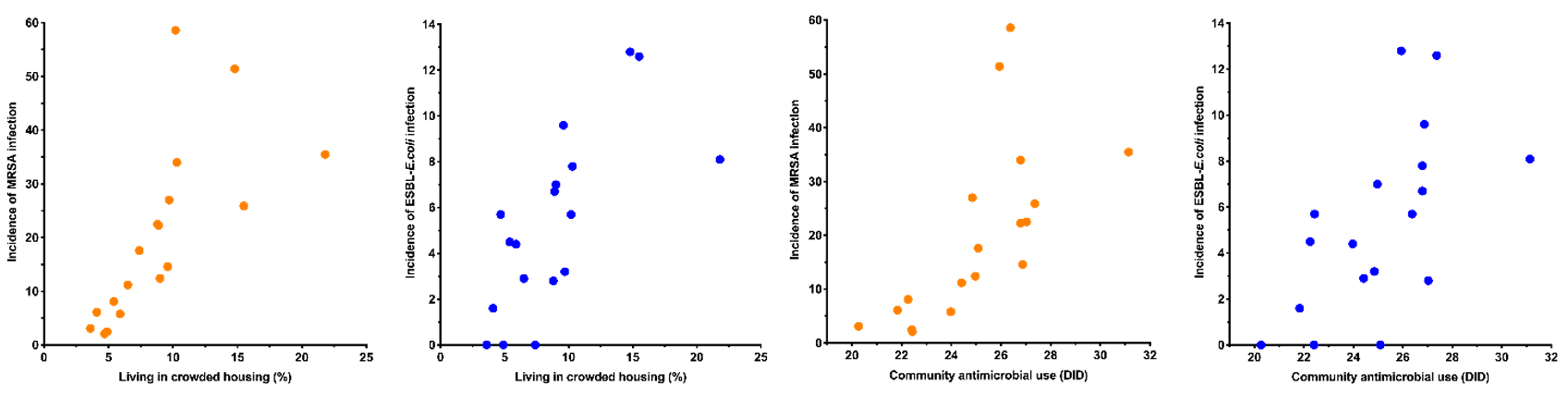
The incidence of ESBL-E. coli infection had a strong positive correlation with the proportion of the population living in crowded housing conditions (Figure 2, Table 2). It also had a moderate positive correlation with the proportion of the population reporting Asian ethnicity; reporting Pacific ethnicity; who were overseas born new arrivals; and with community antimicrobial use (Figure 2, Table 2). ESBL-E. coli infection had a strong negative correlation with the proportion of the population aged >65 years and the proportion reporting European ethnicity (Figure 2, Table 2).
Discussion
Variation in the incidence of antimicrobial resistant pathogen infection is almost certainly caused by multiple factors that promote selective pressure, increase transmission and increase risk of clinical infection.7 In this study we examined the relationship between population-level exposures and the incidence of MRSA and ESBL-E. coli infection among New Zealand DHB populations.
Community antimicrobial use and the proportion of the population living in crowded housing were both strongly to very strongly correlated with MRSA and ESBL-E. coli infection. These variables were also strongly co-correlated (rs=0.85); consistent with a recent study in the Counties-Manukau DHB population that demonstrated household crowding was associated with higher rates of antimicrobial prescribing.18 This co-correlation may be confounding one or other of the variables’ association with MRSA and ESBL-E. coli infection. Nonetheless, the largely clonal nature of MRSA and ESBL-E. coli, means it is plausible that community antimicrobial use may contribute to a higher incidence of infection by rendering people more vulnerable to colonisation and infection in the event of subsequent exposure.19 It is known that community antimicrobial use in New Zealand is high compared to other high-income countries.17,20 Household crowding and antimicrobial use could be part of the same causal pathway (ie, crowded households have more infections and thus require more antimicrobials). However, as these organisms are known to spread between household members, it is likely that crowding also has a more direct effect by promoting transmission of ESBL-E. coli and MRSA when one or more household member is colonised.21,22
Population levels of socioeconomic deprivation had a strong positive correlation with MRSA infection. This relationship may be affected by moderate co-correlation between socioeconomic deprivation and household crowding (rs=0.57) and community antimicrobial use (rs=0.48). Socioeconomic deprivation has been associated with higher levels of antimicrobial use in New Zealand, although not in all regions.20,23 Nonetheless, a direct contribution of deprivation to higher incidences of MRSA infection is considered probable as living in an area of greater socioeconomic deprivation is an established risk factor for MRSA infection and all cause infectious disease morbidity in New Zealand.24–26
The proportion of the population reporting Māori ethnicity and Pacific ethnicity had moderate positive correlations with MRSA infection. MRSA isolation rates have previously been reported to be higher among Māori and Pacific peoples than New Zealand Europeans or Asians.24 These observations are likely to be explained in part by co-correlation with socioeconomic deprivation (Māori), household crowding (Pacific) and antimicrobial use (Pacific). The proportion of the population reporting Asian ethnicity, Pacific ethnicity and overseas-born new arrivals, had moderate positive correlations with the incidence of ESBL-E. coli infection. Co-correlation with each other and confounding by other positively associated variables (Table 1) are possible explanations for these associations. Asian ethnicity has however been previously associated with an individual risk of ESBL-E. coli infection in New Zealand and is presumed to reflect initial acquisition in highly endemic regions of Asia.27 It is possible that despite established ESBL-E. coli endemicity in New Zealand that ongoing importation may still contribute to variation in infection rates between DHBs. European ethnicity had a strong negative correlation with MRSA and ESBL-E. coli infection. This seems likely to largely reflect the summation of a number of negative correlations between European ethnicity and positively associated population variables (Table 1).
The proportion of the population aged <5 years had a strong positive correlation with MRSA infection. This relationship may be affected by co-correlation of age <5 with household crowding, socioeconomic deprivation and antimicrobial use. However, a direct causal relationship is considered a possibility as high rates of MRSA infection are well documented among New Zealand pre-school aged children.24,25 Age >65 years was negatively correlated with ESBL-E. coli infection rates (despite older age typically considered an individual risk factor for infection). This may be explained by the negative correlation of age >65 years with other positively associated variables (Table 1).
This study has a number of limitations. The ecological design precludes causal relationships from being definitively established. Secondly, the limited number of data points available resulted in wide confidence intervals and prevented adjustment for confounding due to co-correlation between variables. Thirdly, inherent limitations in the source data used may have affected our findings. Finally, the results are of uncertain generalisability to settings outside of New Zealand. Our findings require further research to explore the causative relationships, utilising, for instance, population-level data amenable to multivariate analysis, or individual level analysis inclusive of both individual and population-level exposures.
Despite the limitations, our findings provide insight into the potential contribution of population-level exposures to variation in the incidence of MRSA and ESBL-E. coli infection between populations within New Zealand (an MRSA/ESBL-E. coli endemic high-income country). The differences observed between MRSA and ESBL-E. coli suggest that a single set of generic interventions may have a differential impact on different AMR-associated organisms. Moreover, several MRSA and ESBL-E. coli infection associated exposures are in principle modifiable. It may be that policy measures to reduce rates of household crowding and socioeconomic deprivation could present potentially novel approaches to reducing AMR. Similarly, reducing community antimicrobial use in the population at large may help reduce MRSA and ESBL-E. coli infections. As New Zealand and other countries devise and implement national AMR response plans, these findings highlight directions for future research and potentially novel opportunities to reduce the burden of AMR.
Aim
National responses to antimicrobial resistance (AMR) require an understanding of the factors driving its development and spread. Research to date has primarily focused on determining individual-level risk factors for AMR-associated infections. However, additional insights may be gained by investigating exposures associated with AMR variation at the population level.
Methods
We used an ecological study design to describe the association between the incidence rate of methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase producing Escherichia coli (ESBL-E. coli) infection and population-level variables among 18 geographically distinct populations, defined by district health boards, in Aotearoa New Zealand. Associations were described using Spearman’s correlational analysis.
Results
Positive correlations were found between the incidence of both MRSA and ESBL-E. coli infection and household crowding and community antimicrobial use. Positive correlations were also observed between MRSA and socioeconomic deprivation; age <5 years; Māori ethnicity; and Pacific ethnicity. For ESBL-E. coli, positive correlations were also observed with Asian ethnicity; Pacific ethnicity; and overseas-born new arrivals. European ethnicity was negatively correlated with both MRSA and ESBL-E. coli infection.
Conclusion
These findings provide insight into the potential contribution of population-level exposures to MRSA and ESBL-E. coli infection in New Zealand. Exposures such as household crowding, community antimicrobial use and socioeconomic deprivation, are in principle modifiable and may present potentially novel opportunities to reduce the burden of AMR.
Authors
Matthew R Blakiston, Clinical Microbiologist, Microbiology Department, LabPLUS, Auckland District Health Board, Auckland; Joshua T Freeman, Clinical Microbiologist, Microbiology Department, Canterbury Health Laboratories, Canterbury District Health Board, Christchurch.Acknowledgements
The authors acknowledge Helen Heffernan from The Institute of Environmental Science and Research Limited (ESR) for collation and provision of district health board MRSA and ESBL-E. coli infection incidence rate data.Correspondence
Matthew R Blakiston, PO BOX 110031, Auckland City Hospital, Auckland 1148.Correspondence email
mattbl@adhb.govt.nzCompeting interests
Nil.1. Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016; 387:168–75.
2. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ Accessed February 2017.
3. Goossens H, Ferech M, Stichele RV, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005; 365:579–87.
4. Alvarez-Uria G, Gandra S, Laxminarayan R. Poverty and prevalence of antimicrobial resistance in invasive isolates. Int J Infect Dis. 2016; 52:59–61.
5. Bruinsma N, Hutchinson J, van den Bogaard A, et al. Influence of population density on antimicrobial resistance. J Antimicrob Chemother. 2003; 51:385–90.
6. Collignon P, Athukorala P, Senanayake S, Khan F. Antimicrobial resistance: The major contribution of poor governance and corruption to this growing problem. PloS ONE. 2015;10: http://doi.org/10.1371/journal.pone.0116746
7. Holmes A, Moore L, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016; 387:176–87.
8. MacPherson D, Gushulak B, Baine W, et al. Population mobility, globalization, and antimicrobial resistance. Emerg Infect Dis. 2009; 15;1727–32.
9. van der Bij A, Pitout J. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J Antimicrob Chemother. 2012; 67;2090–100.
10. Wellington E, Boxall A, Cross P, Feil E, Gaze W, Hawkey P, et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infec Dis. 2013; 13:155–65.
11. Institute of Environmental Science and Research (ESR). Annual survey of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, 2013. Available at: http://surv.esr.cri.nz/PDF_surveillance/Antimicrobial/ESBL/ESBL_2013.pdf Accessed February 2017.
12. Institute of Environmental Science and Research (ESR). Annual Survey of Methicillin Resistant Staphylococcus Aureus (MRSA), 2013. Available at: http://surv.esr.cri.nz/PDF_surveillance/Antimicrobial/MRSA/MRSA_2013.pdf Accessed February 2017.
13. Statistics New Zealand. 2013 Census District Health Board data tables. Available at: http://m.stats.govt.nz/Census/2013-census/data-tables/dhb-tables.aspx Accessed February 2017.
14. Centre for public health research online. NZ index of deprivation atlas. Available at: http://cphronline.massey.ac.nz/dataviews/report?reportId=260&viewId=96&geoReportId=1619&geoId=15&geoSubsetId= Accessed March 2017.
15. Atkinson J, Salmond C, Crampton P. NZdep2013 Index of Deprivation. Available at http://www.otago.ac.nz/wellington/otago069936.pdf Accessed February 2017.
16. Ministry of Health. Analysis of Household Crowding based on Census 2013 data. Available at: http://www.health.govt.nz/system/files/documents/publications/analysis-of-household-crowding-based-on-census-13-data-dec14-v2.pdf Accessed February 2017.
17. Institute of Environmental Science and Research (ESR). Surveillance report: Antimicrobial consumption in New Zealand 2006-2014. Available at: http://surv.esr.cri.nz/PDF_surveillance/AntibioticConsumption/2014/Antibiotic_Consumption_Report_Final.pdf. Accessed February 2017.
18. Walls G, Vandal A, du Plessis T, et al. Socioeconomic factors correlating with community antimicrobial prescribing. NZ Med J. 2015; 128:16–23.
19. Buffie C, Pamer E. Microbiota-mediated colonisation resistance against intestinal pathogens. Nat Rev Immunology. 2013; 13:790–801.
20. Hobbs M, Grant C, Ritchie S, et al. Antibiotic consumption by New Zealand children: exposure is near universal by the age of 5 years. J Antimicrob Chemother. 2017; 72:1832–40.
21. Knox J, Uhlemann A, Lowy F. Staphylococcus aureus infections: transmission within households and the community. Trends Microbiol. 2015; 23:437–44.
22. Haverkate M, Platteel T, Fluit A, et al. Quantifying within-household transmission of extended spectrum β-lactamase-producing bacteria. Clin Microbiol Infect. 2017; 46: http://doi.org/10.1016/j.cmi.2016.08.021
23. Norris P, Horsburgh S, Keown S, et al. Too much and too little? Prevalence and extent of antibiotic use in a New Zealand region. J Antimicrob Chemother. 2011; 66:1921–1926.
24. Williamson D, Roberts S, Ritchie S, et al. Clinical and molecular epidemiology of Methicillin Resistant Staphylococcus aureus in New Zealand: Rapid emergence of sequence type 5 (ST5)-SCCmec-IV as the dominant community-associated MRSA clone. PloS ONE. 2013;8(4): http://doi.org/10.1371/journal.pone.0062020
25. Williamson D, Lim A, Thomas M, et al. Incidence, trends and demographic of Staphylococcus infections in Auckland, New Zealand, 2001-2011. BMC Infect Dis. 2013;13: http://doi.org/10.1186/1471-2334-13-569
26. Baker M, Barnard L, Kvalsvig A, et al. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet. 2012; 379:1112–19.
27. Freeman J, Williamson D, Heffernan H, et al. Comparative epidemiology of CTX-M-14 and CTX-M-15 producing Escherichia coli: Association with distinct demographic groups in the community in New Zealand. Eur J Clin Microbiol Infect Dis. 2012; 31:2057–60.
