ARTICLE
Vol. 133 No. 1521 |
Estimated inequities in COVID-19 infection fatality rates by ethnicity for Aotearoa New Zealand
The COVID-19 outbreak originated in Wuhan, China before spreading globally to become a pandemic in March 2020. While as of early June 2020, the virus is likely to be eliminated in New Zealand, it is still widespread globally and there is very low domestic immunity.
Full article available to subscribers
The COVID-19 outbreak originated in Wuhan, China before spreading globally to become a pandemic in March 2020. While as of early June 2020, the virus is likely to be eliminated in New Zealand,1 it is still widespread globally and there is very low domestic immunity. There is an ongoing risk of reincursions into New Zealand and planning for these is important. Understanding the potential consequences of future outbreaks with widespread community transmission is crucial to designing and justifying effective measures to prevent this, including border controls, surveillance strategies and social distancing restrictions. Furthermore, better understanding the differential impacts of COVID-19 for high-risk groups within New Zealand, particularly Māori and Pasifika communities, is essential if New Zealand is to appropriately meet the needs of those communities, and mitigate against the effects of existing health inequities.
Obtaining accurate estimates of the risk of fatality is difficult, particularly in the early stages of an epidemic. One reason for this is the difficulty in ascertaining the true number of infections. Testing during an epidemic tends to focus on clinically severe cases, which may bias estimates of fatality rates upwards. Conversely, there is a lag time between onset of symptoms and clinical outcome, which may lead to underreporting of fatalities.2 Fatality rates also depend on factors such as age, pre-existing health conditions and access to healthcare. The case fatality rate (CFR) is the ratio of the number of fatalities to the number of diagnosed cases, whereas the infection fatality rate (IFR) is the ratio of the number of fatalities to the total number of infections. Note although some authors argue that these quantities are ratios and not rates, we use the term fatality rate because it is more commonly used in epidemiology. The CFR is easier to calculate but often less useful than the IFR, which is independent of testing regimes and case definitions.
In this study, we estimate potential inequities in COVID-19 IFRs in New Zealand by ethnicity in the event that a future reincursion of COVID-19 leads to widespread community transmission. Fortunately, the number of cases in New Zealand to date has been too small to provide a sufficient sample size to stratify by ethnicity and age. Therefore, we project international age-stratified data on COVID-19 IFR2 onto New Zealand’s population, accounting for age structure and the effect of major comorbidities by ethnicity. The international IFR data was derived using a robust statistical approach, accounting for case under-ascertainment and right censoring.2 It is consistent with more recent evidence from international studies3 and serological surveys4,5 which point to a population-level IFR between 0.5% and 1%. Using this data avoids the need to make assumptions around case ascertainment rates or total number of infections in New Zealand. Nevertheless, as the age-stratified IFR can vary between populations, our results should be viewed in a relative sense for comparing ethnicities rather than a precise prediction of the absolute value of IFR. The methodology we present could also be useful in the future if similar novel infectious diseases arrive in New Zealand and cannot be contained.
We adjust our estimates to account for the fact that, although Māori and Pacific populations are structurally younger than other ethnic groups, they have shorter life expectancy and higher rates of premature death at all ages. Mortality rates for older Māori are shaped by their life course, which includes increased exposure to infectious disease and conditions affecting respiratory function.6 We also adjust for inequity in unmet healthcare need, which captures some of the structural biases and racism within the healthcare system.7,8 We discuss other factors, not reflected in official data, which could further increase IFR for high-risk communities. These increased risk factors, and the adjustments made to model them, critically acknowledge the historic and contemporary differential experiences of exposure to, infection with, transmission of, and treatment for infectious and chronic disease for Māori.9 During the 1918 influenza pandemic, Māori death rates were seven times higher than those for New Zealand European/Pākehā. As recently as 2009, during the H1N1 influenza pandemic, rates of infection for Māori were twice that of Pākehā, with increased severity.10 The prevailing impacts of colonisation, resulting in historically under-served communities, provide key contexts for the need to understand IFR by ethnicity for New Zealand.
IFR is only one aspect of the epidemiology of COVID-19 and other factors, such as COVID-19 incidence and reduced access to healthcare services during a pandemic, could also contribute to inequities in overall health burden. We focus on IFR because it provides a key indication of how the severity of COVID-19 could vary by ethnicity, which will help identify high-risk communities. In addition, IFR is an important input for models of COVID-19 spread and mortality.11 However, it will be important to refine these models to account for ethnicity-specific differences in other factors, including incidence and access to healthcare.
To date, there has been little quantitative analysis on the effects of ethnicity for COVID-19 in New Zealand. Given the speed at which COVID-19 can spread, there is an urgent need to prepare healthcare services and establish measures to protect at-risk groups. To address this, we use a simplified and approximate methodology, which contains numerous limitations (see Discussion). There are also shortcomings in the data on which our estimates are based, which make it difficult to disentangle the effects of age and comorbidity. Our results are an initial guide to the potential scale of COVID-19 inequity in New Zealand rather than a prediction of absolute IFR.
Methods
Data
Tables 1–2 show data on the age structure (2018 census, usual resident population13) for Māori, Pacific and New Zealand European/other, life expectancy for Māori, Pacific and non-Māori,12 and international data on age-specific COVID-19 IFRs.2 We chose to use this IFR data because it was stratified by age and included robust controls for under-ascertainment of cases and right-censoring. New Zealand European/other population statistics were estimated by subtracting the sum of Māori and Pacific populations from the total. Table 3 shows data on the prevalence of diabetes, heart disease, asthma, cancer and smoking by ethnicity in New Zealand.14–18 The health data uses a mixture of prioritised ethnicity and total response classifications, we do not expect this to have a significant effect on the final results. Table 4 shows data on relative case fatality rate (CFR) for these conditions from China CDC.19 Hypertension has not been classified by New Zealand district heath boards as a high-risk condition20 so we did not include hypertension in our analysis. This is supported by a recent study from the UK, which found that hypertension was not associated with higher risk of fatality after controlling for other comorbidities.21 Other chronic conditions such as renal disease may also have a significant effect,21 but these were not included in the China CDC study.19 In the absence of data on these health conditions collected using a consistent study design, we therefore excluded these from our study.
Adjusting for life expectancy
Māori typically experience adverse health outcomes at an earlier age than non-Māori.22 To reflect this, we adjusted the age-specific IFR estimates2 by the most recent (2012–14) estimates of life expectancy for each ethnicity. This approach is consistent with international evidence that COVID-19 mortality is approximately proportional to total mortality, meaning that COVID-19 amplifies existing mortality risk evenly for different groups.23 The gap in life expectancy is different for male and female and for different age groups. For simplicity, we used an average of the male and female life expectancy gap for the youngest age cohort. We calculated the IFR for age group , adjusted for the life expectancy of ethnicity group j, as
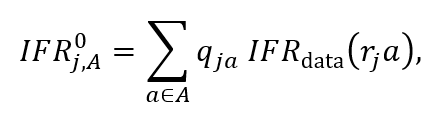
where qja is the proportion of ethnicity j within age group A that is age a, IFRdata(a) is the IFR at age in the reference population (in which the IFR data were measured), and rj is the ratio of the life expectancy of the reference population to the life expectancy of group j. We used 20-year age brackets to match the New Zealand health data, but Eq. (1) accounts for the distribution of ages within each age bracket for each ethnicity. IFRdata was evaluated at ages rja by linearly interpolating between the midpoints of the age brackets.2 The midpoint for the 80+ age group was set at 85, with the IFR for all ages >85 fixed at this rate.
Adjusting for unmet healthcare need
There is evidence from the UK that groups with greater socioeconomic deprivation and black, Asian and minority ethnic groups, after controlling for age and comorbidities, have higher fatality risk.21 These effects are difficult to quantify for New Zealand and no direct data is available. To capture some of this effect, we used data on unmet healthcare needs as a rough proxy for under-reporting of comorbid conditions and other inequities (see Discussion). The proportion of people who reported being unable to see a GP when needed (uj) was 41.4% for Māori, 35.9% for Pacific and 30.1%16 for New Zealand European/other. We weighted IFRs for each ethnicity by these values.
Adjusting for comorbidity
We calculated relative risk factors Ck (Table 4) for each comorbid condition as:
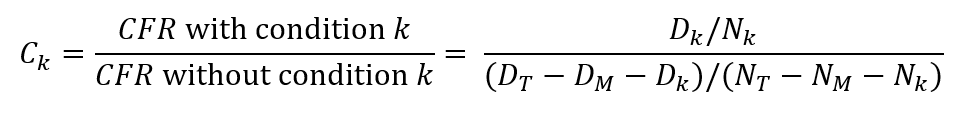
where Dk is the number of deaths in patients with condition k and Nk is the number patients with condition k.19 Subscripts T and M respectively represent the same quantities for the total sample and for those with missing data.
To account for effect of comorbidity, we made several simplifying assumptions:
1. The overall population IFR in New Zealand across all ethnicities is approximately equal to the overall average IFR estimates from China2 (see Discussion).
2. Conditions are independent so P(condition 1 and condition 2) = P(condition 1)*P(condition 2).
3. Individuals with multiple conditions experience the product of the risk factors of each condition, ie, there are no interaction effects between conditions.
4. The relative effect of comorbidities on IFR is the same as the measured effect on CFR19 and is not age specific.
This allowed us to define a comorbidity weighting factor for ethnicity j and age group A as:
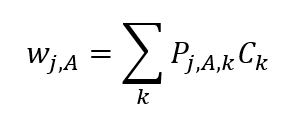
where Pj,A,k is the proportion of ethnicity j and age with condition k.
Accounting for the combined effects of age and comorbidity is not straightforward, as we only had data on the overall effect of each comorbidity rather than age-specific effects. Prevalence of comorbid conditions, such as heart disease, will be higher in groups with older populations. This is already reflected, to some extent, in the age distribution of IFR (Table 1). Therefore, taking an age-structured IFR and adjusting for comorbidity will over-account for the effects of age-related health conditions. Similarly, adjusting for differences in life expectancy and prevalence of comorbid conditions will also result in some over-accounting. Conversely, ignoring age structure and only adjusting for selected comorbidities may ignore some age-related effects, for example from conditions that are not in the dataset or age effects that are not linked to a specific health condition (see Discussion).
Table 1: International data on age-specific COVID-19 IFR2 and age distribution of Māori Pacific and New Zealand European/other ethnicity groups in New Zealand.12
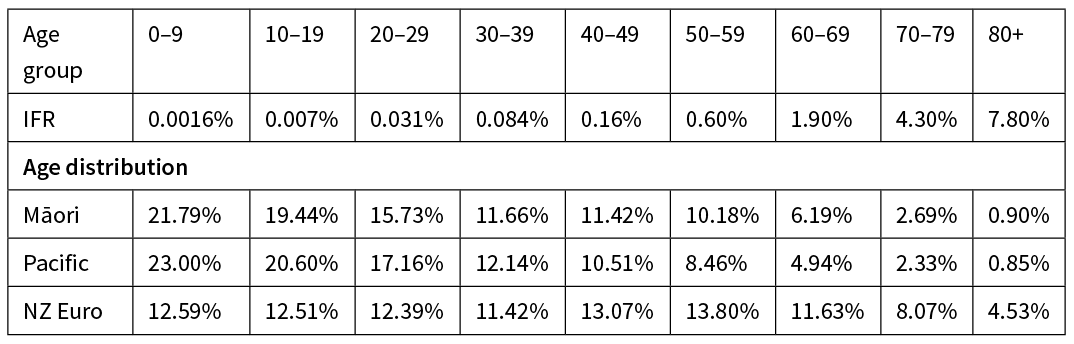
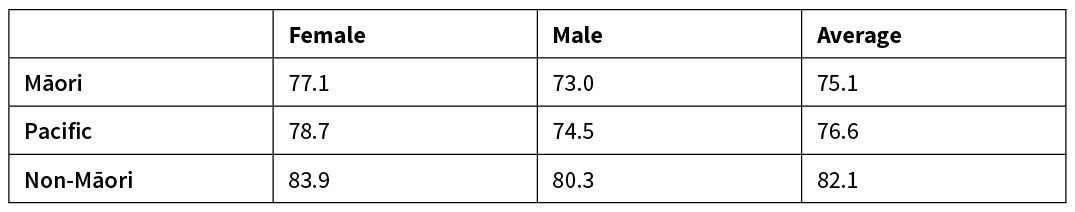
Table 2: Life expectancy at birth (in years) of Māori, Pacific and non-Māori ethnicities.12
We therefore calculated IFRs using two different methods: (i) starting with an age-specific baseline IFR; and (ii) starting with the same population-wide baseline IFR. For each method, we then adjusted the baseline IFR by ethnicity for life expectancy, unmet healthcare need, and comorbidity. Reality lies somewhere between (i) and (ii), so this gives an indicative range for the scale of relative differences in IFR by ethnicity. For method (i), we calculated IFR for age group A and ethnicity j as:
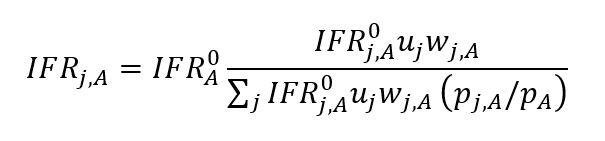
where IFROA is the population average IFR of age group A, IFROj,A is the life-expectancy-adjusted IFR from Eq. (1), and pj,A is the proportion of the population that is in age group A and ethnicity j. The denominator of Eq. (3) normalises so that the overall average IFR in the age group is IFROA. For method (ii), we calculated the overall IFR for ethnicity j as:
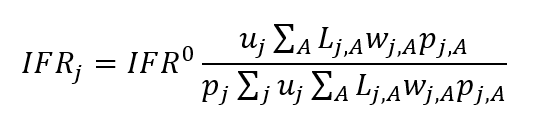
where Lj,A is a factor adjusting for the effect of life expectancy on the IFR for ethnicity j and age group A. The denominator of Eq. (4) normalises so that the overall population average IFR is fixed at IFRO.
Results
The estimated overall population IFR is 0.81%, which is consistent with results from international studies placing the population-level IFR between 0.5% and 1%.3–5 This overall rate could be influenced by numerous factors not accounted for here (see Discussion). The observed case fatality rate (CFR) may be substantially higher than the infection fatality ratio due to asymptomatic infections and case under-ascertainment.2 As of early June 2020, New Zealand’s CFR is around 1.5%. To be consistent with an IFR of 0.81% would imply that 46% of all infections were either asymptomatic or otherwise undiagnosed. This is plausible in light of studies pointing to high rates of asymptomatic infection.24–26 It is also consistent with CFRs and case under-ascertainment rates in the international data.2 Nevertheless, the results shown here should be interpreted primarily as indicating relative differences in IFR across ethnicities and age groups, rather than exact predictions of absolute IFR.
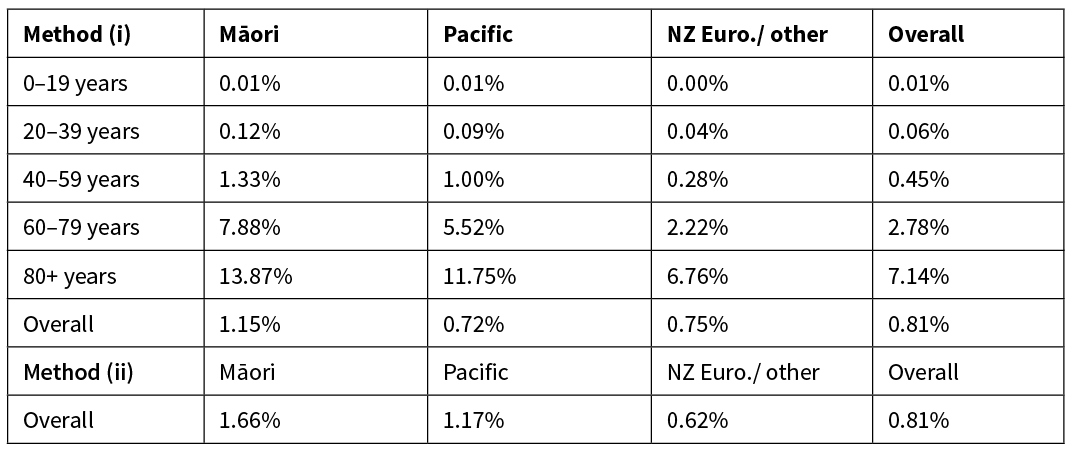
The New Zealand European/other population is structurally old, but has relatively high life expectancy and low unmet healthcare need. Māori and Pacific populations are structurally younger, but have lower life expectancy, higher unmet healthcare need and higher prevalence of comorbid conditions, such as diabetes and asthma. These factors have opposing effects on the IFR and it is difficult to predict whether age, or other covarying factors, is more important. There is little direct evidence to distinguish these effects for COVID-19. We therefore used two methods: method (i) in which IFRs were pre-adjusted for age and method (ii) where they were not. Regardless of which method is used, Māori have a higher IFR than non-Māori (Table 5 and Figure 1). If age is the dominant variable, the estimated IFR for Māori is about 50% higher than for New Zealand European/other. If underlying health conditions (which correlate with age) are more important than age per se, the estimated IFR for Māori is more than 2.5 times higher than New Zealand European/other, and the IFR for Pacific people is almost double that of New Zealand European/other. Recent evidence suggests that age is the dominant factor with comorbidities having smaller though still statistically significant effects.21 This suggests that IFRs are likely to the results from method (i) than to method (ii).
Table 3: Data on prevalence by ethnicity and age of four health conditions and smoking.14–18
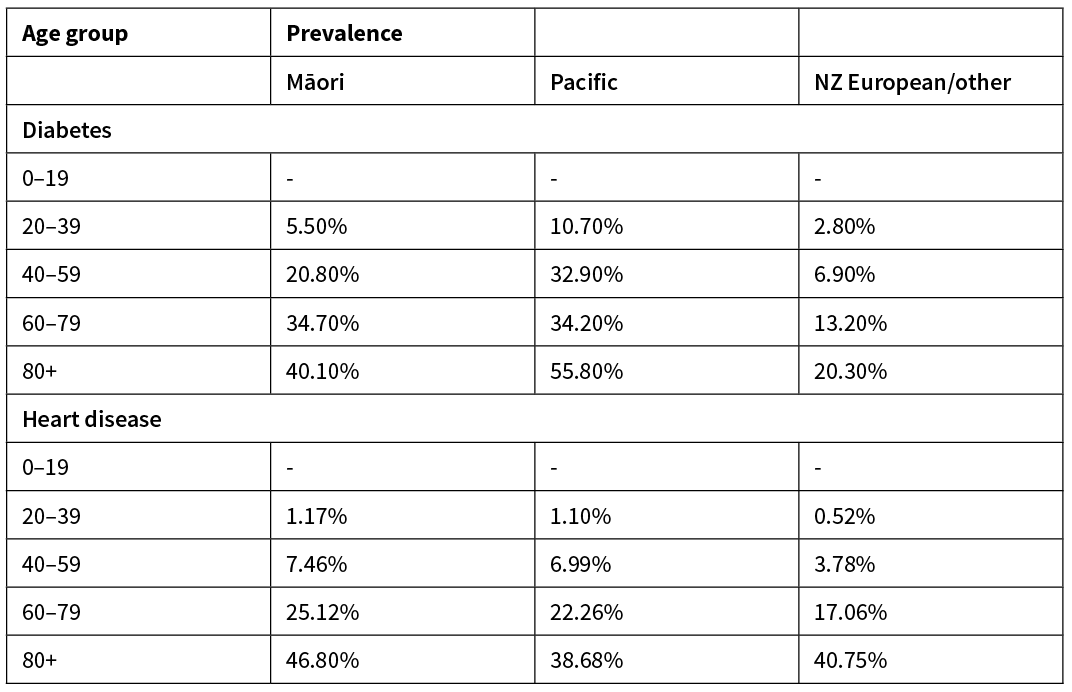
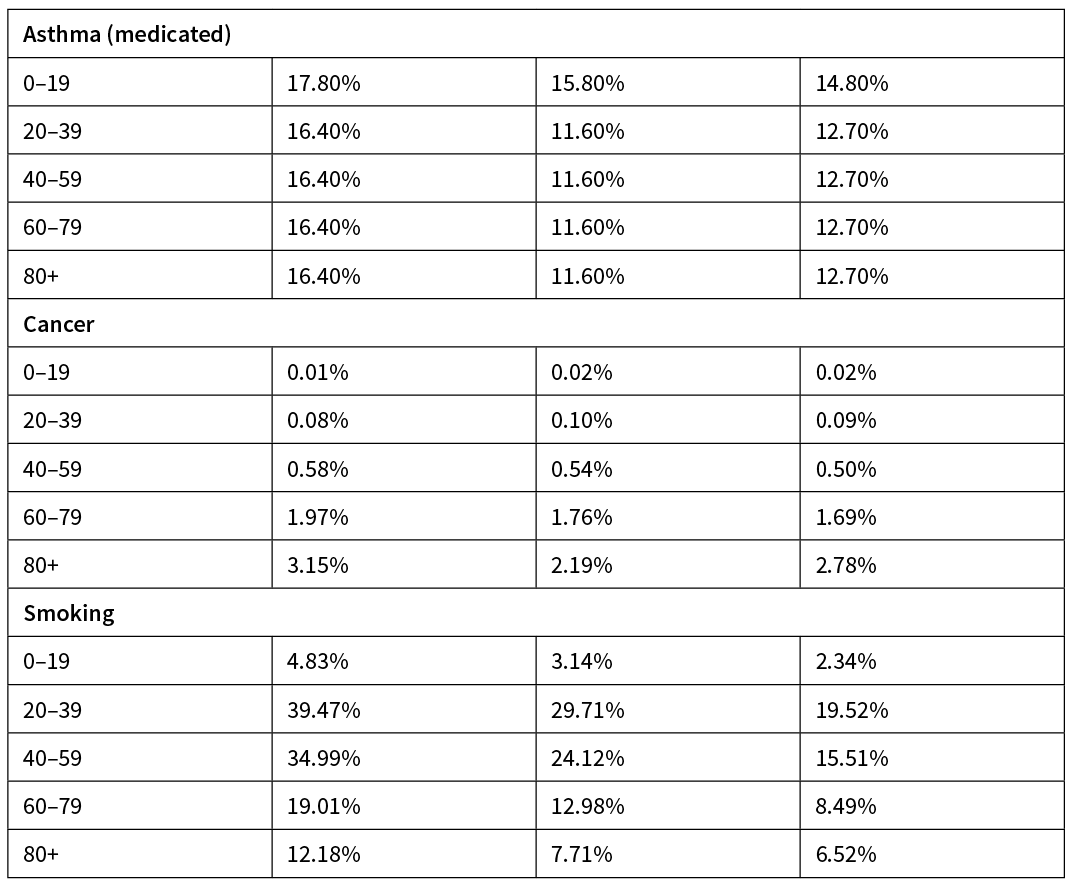
These prevalence data were standardised to 20-year age brackets by making the following approximations. In cases where data were more finely stratified (cancer, smoking), we calculated a weighted average for the prevalence in 20-year bands. The diabetes data were assigned to the closest age bracket (eg, 25–44-year-old diabetes rates were assigned to the 20–39 age bracket). The asthma data were reported in two age brackets: under 15 and 15+; the former was applied to the 0–19 age group and the latter to the others. There were no data on smoking rates for under 15-year olds so the rate was assumed to be zero. This is clearly an underestimate but this will little impact as IFR for COVID-19 is very low in this age bracket.
Table 4: Data on COVID-19 case fatality rates for four comorbidities27 and calculated relative risk factors (Ck). Data were unavailable on the effect of smoking on CFR so we used the incidence of severe cases as a proxy.28
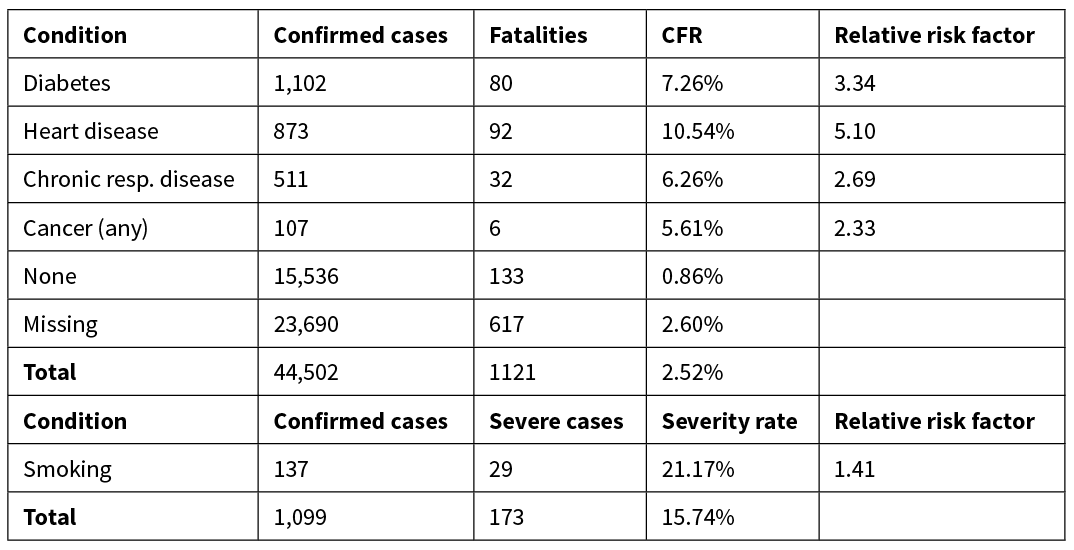
Table 5: Estimated infection fatality rates for each ethnicity group. If age itself is the primary factor, then the results from method (i) are likely to be more accurate. If the age effect is driven by the increase in comorbidity rates with age, the results from method (ii) are likely to be more accurate.
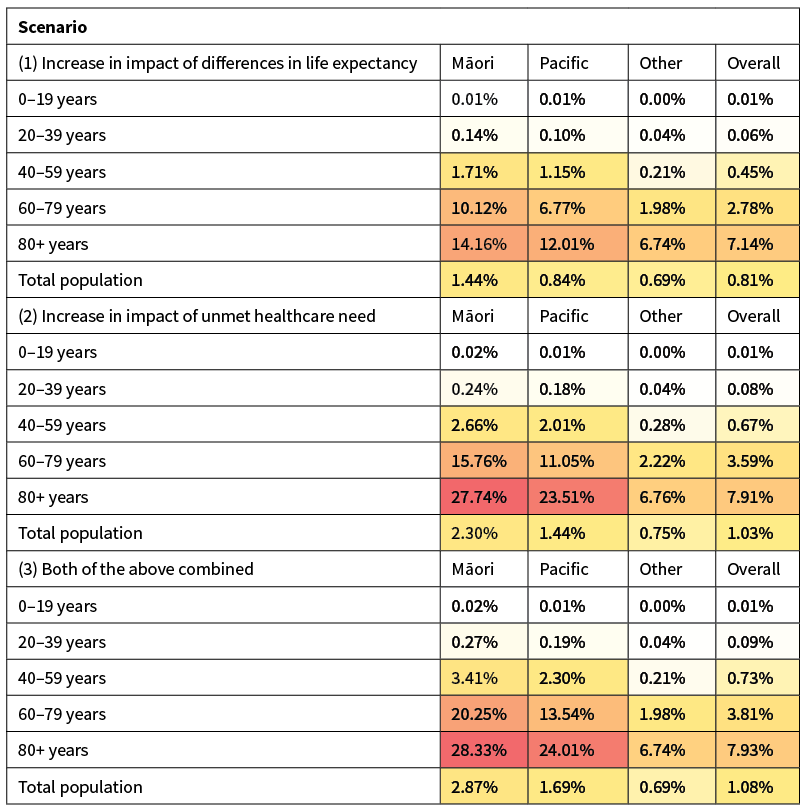
We performed a sensitivity analysis on two model assumptions: the magnitude of the difference in age-specific health outcomes between Māori, Pacific and New Zealand European/other; and the magnitude of the disparity in unmet healthcare need. The estimates we have used for these effects are based on indirect or proxy data (life expectancy and GP access respectively), which are likely to be underestimates. Table 6 shows three scenarios: (1) the impact of the difference in life expectancy (rj in Eq. (1)) between New Zealand European/other and Māori/Pacific people is doubled from 8.6% to 17.2%; (2) the discrepancy in unmet healthcare need between New Zealand European/other and Māori/Pacific people is doubled; and (3) both adjustments. These scenarios reflect a plausible additional level of inequity that may be present. This additional inequity may result in Māori people experiencing fatality rates up to four times greater than New Zealand European/other.
Figure 1: Estimated infection fatality rates by age and ethnicity using method (i). These estimates are adjusted for age structure, relative life expectancy, unmet healthcare need and comorbidity (first section of Table 5).
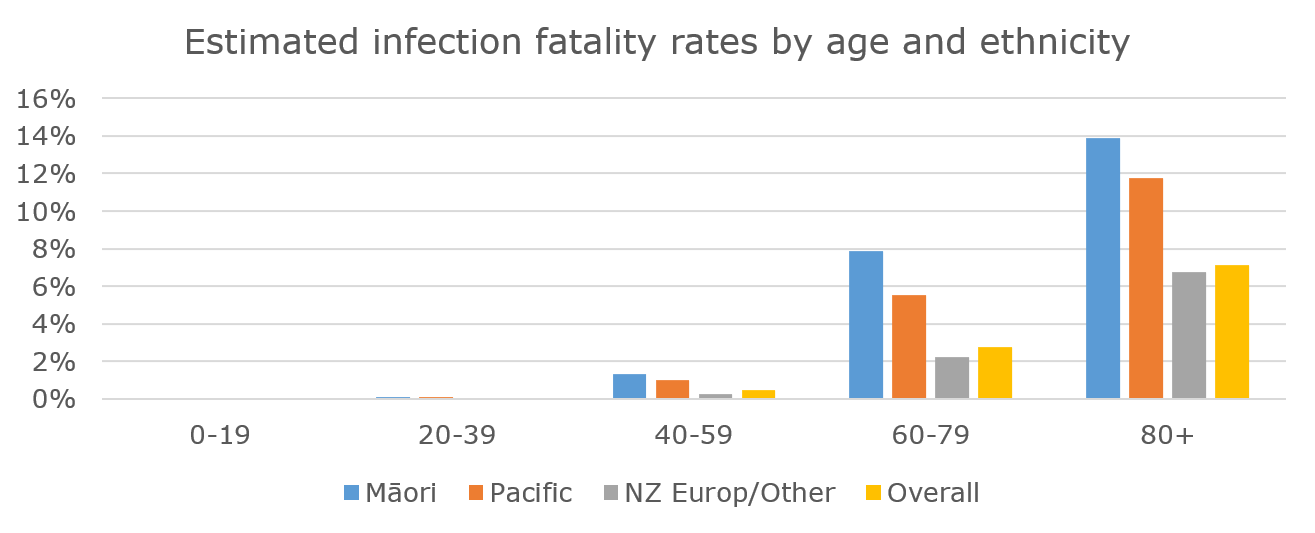
Table 6: Results of sensitivity analysis of the estimated infection fatality rates on assumptions about inequities in healthcare outcomes at a given age. IFRs are pre-adjusted for age (method (i)). Darker colours indicate higher rates. For scenario (1), the change in impact of life expectancy is assumed to redistribute the rates without changing the overall IFR. In scenario (2) and (3), the increase in unmet healthcare needs is assumed to increase the overall IFR.
Discussion
Disentangling the effects of age structure and comorbidity on COVID-19 infection fatality rates is difficult because most studies have been limited to univariate analysis. Estimates from China of the impacts of comorbid conditions are not stratified by age.19 The list of health conditions impacting on COVID-19 infections continues to be expanded as the pandemic develops. The data from which the baseline IFRs used in the current analysis were calculated were adjusted for under-reporting, bias towards more severe cases, and lag time from onset to clinical outcome,2 but may be affected by other biases. The baseline IFRs in our analysis are based on data from China, but there will be country-specific variations in IFR, and potentially higher IFRs in countries with large ethnic minority or Indigenous populations. It is also possible that the IFR may decrease over time as we develop improved treatments. The results discussed here should be treated as a preliminary estimate of relative inequity by ethnicity, rather than predictions of the absolute IFR.
We calculated IFRs using two different methods, giving an indicative range for the scale of potential inequity in IFRs between ethnicities. We adjusted IFRs for differences in life expectancy, unmet healthcare need and prevalence of comorbid conditions. This methodology should be refined over time, particularly as more data become available on outcomes from COVID-19 cases in New Zealand. An alternative approach would be to use standardised metrics such as disability-adjusted life year (DALY) and years lost due to disability (YLD) to infer IFRs by age and ethnicity in New Zealand from the Chinese data. This approach should be investigated, although it is possible that the true magnitude of inequities are not captured in these metrics and the data from which they are derived, so there is a risk that this will underestimate the health burden for Māori and Pacific people.
There are multiple reasons why inequities could end up being larger than estimated here. Hospitalisation and fatality rates for Māori and Pacific people from pandemic H1N1 influenza in 2009 were significantly higher than for New Zealand European.10,29 Māori are more likely to experience multi-morbidity and if the effect of multiple underlying health conditions is worse than simply multiplicative as assumed here, this will increase the IFR for Māori. These disparities could be wider still if differences in age-specific health outcomes and unmet healthcare need are larger than captured in official data. Data on prevalence of comorbid conditions among Māori and Pacific people (Table 3) may be influenced by underreporting, which would make their IFRs higher than calculated here. Avoidable hospitalisations are higher for Māori and Pacific populations,22,30 reflecting broader and more complex structural disadvantage. There exists other widely reported racism within the healthcare system22,31,32 that is not reflected in the available data.
Some of these factors may be less important while COVID-19 case numbers are low, the goal is elimination or containment, and surveillance and contact tracing capacity is adequate. However, if rapid community transmission of COVID-19 takes hold, as has happened elsewhere, it will place unprecedented stress on the healthcare system. This will make access to healthcare increasingly difficult and necessitate decisions by practitioners about who gets access to care. This will almost certainly amplify existing racism in the healthcare system. For example, if triage decisions are based on existence of underlying health conditions, this will automatically disadvantage Māori further. Similar concerns about the inequitable impacts of prioritisation tools have been raised elsewhere.9 Transparency is needed in the risk factors and weightings used to guide decision-making about healthcare service provision, and independent oversight by at-risk groups likely to be disparately impacted by these.
COVID-19 is likely to be more severe in regions or communities with a relatively old population, which is one of the biggest factors affecting hospitalisation and fatality rates. Rural Māori communities have an older age distribution than Māori as a whole33 and have higher unmet healthcare need, so this is a particularly high-risk group. Reported COVID-19 fatalities do not capture indirect impacts, for example deaths attributed to underlying conditions, but precipitated or hastened by COVID-19 infection. These indirect impacts are also likely to fall disproportionately on Māori and Pacific peoples due to higher prevalence of comorbid conditions.
A report from the UK suggests black, Asian and minority ethnic groups are at higher risk from COVID-19 than white majority groups.34 Reports from the US suggest similar trends, where African-American communities are bearing a disproportionate health burden from COVID-19.35 These at-risk communities typically have higher prevalence of underlying health conditions, are more likely to live in overcrowded and multi-generational households, and have relatively young populations.36 Similar factors apply to Māori in New Zealand37 and this reinforces the need to account for the multitude of factors behind inequity, rather than crudely using age structure alone to estimate IFR. The methodology we have used is a first attempt at addressing this. Data on COVID-19 incidence and outcomes in the context of ethnic minority or Indigenous populations that experience inequities in health and healthcare is currently scarce. Making robust comparisons and informing interventions to eliminate inequitable outcomes requires not only more data, but data that is accessible to decision makers in a timely fashion. This reinforces the importance of systematic, comprehensive and timely data collection in New Zealand in order to manage this and any future epidemics.
This study has focused on the infection fatality rate, which does not account for potential differences in transmission and incidence by ethnicity. Risk factors for accelerated transmission include crowded housing, which affects approximately 25% of Māori and 45% of Pacific people.38,39 In addition, multi-generational households increase the risk of transmission to older groups. These compounding factors mean that Māori and Pacific peoples are at risk of bearing a disproportionate health burden from COVID-19. A comprehensive analysis of these factors is outside the scope of this work. It will be critical to incorporate these into disease transmission models that are used to inform New Zealand’s COVID-19 response.40 This will enable the combined effect of incidence and IFR to be more accurately measured and to inform effective strategies that recognise the diversity of higher-risk groups, communities and regions.
Aim
There is limited evidence as to how clinical outcomes of COVID-19 including fatality rates may vary by ethnicity. We aim to estimate inequities in infection fatality rates (IFR) in New Zealand by ethnicity.
Results
The IFR for Māori is estimated to be 50% higher than that of non-Māori, and could be even higher depending on the relative contributions of age and underlying health conditions to mortality risk.
Conclusion
There are likely to be significant inequities in the health burden from COVID-19 in New Zealand by ethnicity. These will be exacerbated by racism within the healthcare system and other inequities not reflected in official data. Highest risk communities include those with elderly populations, and Māori and Pacific communities. These factors should be included in future disease incidence and impact modelling.
Authors
Nicholas Steyn, School of Mathematics and Statistics University of Canterbury; Department of Physics, University of Auckland; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Rachelle N Binny, Manaaki Whenua; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Kate Hannah, Department of Physics, University of Auckland; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Shaun C Hendy, Department of Physics, University of Auckland; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Alex James, School of Mathematics and Statistics University of Canterbury; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Tahu Kukutai, University of Waikato, Hamilton; Audrey Lustig, Manaaki Whenua; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Melissa McLeod, Department of Public Health, University of Otago; Michael J Plank, School of Mathematics and Statistics University of Canterbury; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Kannan Ridings, Department of Physics, University of Auckland; Te Pūnaha Matatini: the Centre for Complex Systems and Networks; Andrew Sporle, Department of Statistics, University of Auckland; McDonaldSporle Ltd., Auckland.Acknowledgements
The authors would like to thank Tony Blakely, Nigel French, Ricci Harris, Karen Wright, Thomas Lumley, Collin Tukuitonga and five anonymous reviewers for helpful comments on the manuscript.Correspondence
Michael J Plank, School of Mathematics and Statistics, University of Canterbury, Christchurch 8140.Correspondence email
michael.plank@canterbury.ac.nzCompeting interests
Dr Binny, Ms Hannah, Dr James, Dr Plank, Mr Steyn, Dr Hendy and Dr Lustig report grants from Te Pūnaha Matatini during the conduct of the study. Dr Kukutai is a member of the Chief Science Advisor Forum and incoming Director of Ngā Pae o Te Māramatanga (pending successful bid).1. Binny RN, Hendy SC, James A, et al. Probability of elimination for COVID-19 in Aotearoa New Zealand. 2020. Retrieved from http://www.tepunahamatatini.ac.nz/2020/06/06/probability-of-elimination-for-covid-19-in-aotearoa-new-zealand/
2. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet. 2020, http://doi.org/10.1016/S1473-3099(20)30243-7
3. Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection-fatality rates. MedRxiv. 2020, DOI:10.1101/2020.05.03.20089854
4. Salje H, Kiem CT, Lefrancq N, et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020, DOI 10.1126/science.abc3517.
5. Stringhini S, Wisniak A, Piumatti G, et al. Repeated seroprevalence of anti-SARS-CoV-2 IgG antibodies in a population-based sample from Geneva, Switzerland. medRxiv preprint. 2020, DOI 10.1101/2020.05.02.20088898.
6. Yon Y, Crimmins EM. Cohort Morbidity Hypothesis: Health Inequalities of Older Māori and non-Māori in New Zealand. New Zealand population review. 2014; 40:63–83.
7. Robson B, Harris R (eds). Hauora: Māori Standards of Health IV. A study of the years 2000–2005. Wellington: Te Rōpū Rangahau Hauora a Eru Pōmare. 2007, Retrieved from http://www.otago.ac.nz/wellington/departments/publichealth/research/erupomare/research/otago019494.html
8. Waitangi Tribunal. Hauora: Report on Stage One of the Health Services and Outcomes Kaupapa Inquiry. WAI 2575, Waitangi Tribunal Report. Legislation Direct, Lower Hutt, New Zealand. 2019, Retrieved from http://waitangitribunal.govt.nz/inquiries/kaupapa-inquiries/health-services-and-outcomes-inquiry
9. King P, Cormack D, McLeod M, et al. COVID-19 and Māori health – when equity is more than a word. 2020, Retrieved from http://blogs.otago.ac.nz/pubhealthexpert/2020/04/10/covid-19-and-Maori-health-when-equity-is-more-than-a-word/#more-4012
10. Wilson N, Telfar-Barnard L, Summers J, et al. Differential mortality by ethnicity in 3 influenza pandemics over a century, New Zealand. Emerg Infect Dis. 2012; 18:71–77.
11. Ferguson N, Laydon D, Nedjati-Gilani G, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College. 2020, Retrieved from: http://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf
12. StatsNZ. Infoshare. Retrieved March 19, 2020, from http://archive.stats.govt.nz/infoshare/
13. StatsNZ. Census 2018. Retrieved March 19, 2020 from http://archive.stats.govt.nz/infoshare/
14. Chan WC, Wright C, Riddell T, et al. Ethnic and socioeconomic disparities in the prevalence of cardiovascular disease in New Zealand. New Zealand Medical Journal. 2020; 121:11–20.
15. Coppell KJ, Mann JI, Williams SM, et al. Prevalence of diagnosed and undiagnosed diabetes and prediabetes in New Zealand: findings from the 2008/09 Adult Nutrition Survey. The New Zealand Medical Journal. 2013 Mar; 126(1370):23–42.
16. Ministry of Health New Zealand. 2018/19 New Zealand Health Survey Annual Data Explorer. Retrieved March 19, 2020, from http://minhealthnz.shinyapps.io/nz-health-survey-2018-19-annual-data-explorer
17. StatsNZ. Census 2013. 2013, Retrieved March 19, 2020 from http://archive.stats.govt.nz/infoshare/
18. Zhang J, Barnard L. The impact of respiratory disease in New Zealand: 2018 update. Asthma and Respiratory Foundation of NZ. 2018, Retrieved from http://s3-ap-southeast-2.amazonaws.com/assets.asthmafoundation.org.nz/images/NZ-Impact-Report-2018_FINAL.pdf
19. China CDC. Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19). Weekly China CDC. 2020, Retrieved from http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51
20. New Zealand Government. COVID 19 - Self-identification and self-assessment of underlying health issues (v1.0). Retrieved 26 March 2020, from http://mcusercontent.com/d2dc982e906db23c1d25a9718/files/36cb9457-c3cc-4176-bca3-569befa35c58/Document_2_covid_19_24_3.pdf
21. Williamson E, Walker A J, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. Nature. 2020, http://doi.org/10.1038/s41586-020-2521-4
22. Ministry of Health New Zealand. Wai 2575 Māori Health Trends Report. Wellington: Ministry of Health. 2020, Available from http://www.health.govt.nz/publication/wai-2575-Māori-health-trends-report
23. Spiegelhalter D. Does Covid raise everyone’s relative risk of dying by a similar amount? More evidence. A report from the Winton Centre for Risk and Evidence Communication at Cambridge. Retrieved 15 April 2020 from http://medium.com/wintoncentre/does-covid-raise-everyones-relative-risk-of-dying-by-a-similar-amount-more-evidence-e7d30abf6821
24. Byambasuren O, Cardona M, Bell K, Clark J, et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. medRxiv preprint. 2020, DOI 10.1101/2020.05.10.20097543.
25. Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. medRxiv preprint. 2020, DOI 10.1101/2020.04.17.20053157.
26. Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus 1 infections (COVID-19). International Journal of Infectious Diseases. 2020; 94:154–155.
27. CDC. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States, February 12–March 16, 2020. Morbidity and Mortality Weekly Report. 2020, Retrieved from http://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm
28. Guan W, Ni Z, Yu Hu, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England Journal of Medicine. 2020, DOI:0.1056/NEJMoa2002032
29. Verrall A, Norton K, Rooker S, et al. Hospitalizations for pandemic (H1N1) 2009 among Māori and Pacific Islanders, New Zealand. Emerg Infect Dis. 2010; 16:100–2.
30. Basu A, Brinson D. The effectiveness of interventions for reducing ambulatory sensitive hospitalisations: a systematic review. HSAC Report. 2008; 1(6).
31. Cormack D, Harris R, Stanley J, et al. Ethnic bias amongst medical students in Aotearoa/New Zealand: Findings from the Bias and Decision Making in Medicine (BDMM) study. PLOS One 2018; 13(8):e0201168
32. Reid R, Taylor-Moore K, Varona G. Towards a Social-Structural Model for Understanding Current Disparities in Māori Health and Well-Being. Journal of Loss and Trauma, 2014; 19:6, 514–536, DOI: 10.1080/15325024.2013.809295
33. Ministry of Health New Zealand. Mātātuhi Tuawhenua: Health of Rural Māori 2012. 2012, Available from http://www.health.govt.nz/system/files/documents/publications/matatuhi-tuawhenu-health-of-rural-maori-2012.pdf
34. ICNARC. ICNARC report on COVID-19 in critical care. 2020, Retrieved from http://www.icnarc.org/DataServices/Attachments/Download/76a7364b-4b76-ea11-9124-00505601089b
35. IDPH. Illinois Department of Public Health COVID-19 Statistics. 2020, Retrieved from http://www.dph.illinois.gov/covid19/covid19-statistics
36. UK Government. Overcrowded households. 2018, Retrieved from http://www.ethnicity-facts-figures.service.gov.uk/housing/housing-conditions/overcrowded-households/latest#by-ethnicity-and-socio-economic-group
37. McLeod M, Gurney J, Harris R, et al. COVID-19: we must not forget about Indigenous health and equity. Aust NZ J Public Health. 2020. DOI 10.1111/1753-6405.13015
38. Schluter P, Carter S, Kokaua J. Indices and perception of crowding in Pacific households domicile within Auckland, New Zealand: findings from the Pacific Islands Families Study. New Zealand Medical Journal. 2007; 120:1248.
39. Stats NZ. Living in a crowded house: Exploring the ethnicity and well-being of people in crowded households. 2018, Retrieved from http://www.stats.govt.nz/assets/Uploads/Reports/Living-in-a-crowded-house-exploring-the-ethnicity-and-well-being-of-people-in-crowded-households/living-in-a-crowded-house-exploring-the-ethnicity-and-well-being-of-people-in-crowded-households.pdf
40. James A, Hendy SC, Plank MJ, Steyn N. Suppression and mitigation strategies for control of COVID-19 in New Zealand. MedRxiv. 2020, DOI: 10.1101/2020.03.26.20044677.
